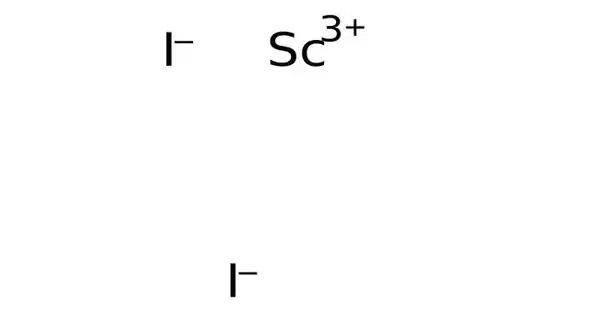Scandium triiodide, also known as scandium iodide, is an inorganic compound with the formula ScI3 and is classified as a lanthanide iodide. This salt is a yellowish powder. It is typically found in the form of a white or colorless crystalline solid and is known for its interesting properties and potential applications in various scientific and industrial fields.
It is used in metal halide lamps together with similar compounds, such as caesium iodide, because of their ability to maximize emission of UV and to prolong bulb life. The maximized UV emission can be tuned to a range that can initiate photopolymerizations.
Reaction
Scandium triiodide reacts with moisture in the air, slowly decomposing to form scandium hydroxide and iodine. It can also react with alkalis to form scandium salts. It is highly reactive and is considered to be hygroscopic, meaning it readily absorbs moisture from the air. The purest scandium triiodide is obtained through direct reaction of the elements:
2 Sc + 3 I2 → 2 ScI3
Alternatively, but less effectively, one can produce anhydrous scandium triiodide by dehydrating ScI3(H2O)6.
Properties
Scandium tri iodide typically appears as a white or colorless crystalline solid, though it may appear off-white due to the influence of impurities. It has a relatively high melting point of around 650°C (1202°F). It is soluble in water and forms a colorless solution when dissolved. It is also soluble in alcohol and acetone.
- Chemical formula: ScI3
- Molar mass: 425.66
- Appearance: Yellowish solid
- Melting point: 920 °C (1,690 °F; 1,190 K)
Natural Occurrence
Scandium tri iodide does not occur naturally as a pure compound. Scandium, the metal from which it is derived, is found in trace amounts in minerals like thortveitite (Scandium silicate) and bazzite. These minerals are typically found in a few locations, including Norway, Madagascar, and the USA. Iodine, the other component of ScI₃, is derived from natural sources such as seawater and certain minerals like caliche and iodine-rich brines.
Applications
While scandium and its compounds, including scandium triiodide, are not widely used on a commercial scale, they hold promise in certain niche applications:
- Research and Development: ScI₃ has potential use in scientific research, particularly in areas like semiconductor technology, crystal growth, and materials science.
- Scandium Alloys: Scandium is used in small amounts in aluminum alloys, improving their strength, durability, and resistance to corrosion. However, scandium tri iodide itself does not have a direct role in this industry.
- Energy Applications: Due to scandium’s properties, ScI₃ and other scandium compounds may have future applications in energy storage, high-performance materials, or as catalysts.
Safety Considerations
- Scandium triiodide, like other iodides, can be harmful if ingested, inhaled, or if it comes into contact with the skin in significant quantities.
- Care should be taken to handle it in controlled environments, such as a fume hood, with appropriate protective gear.