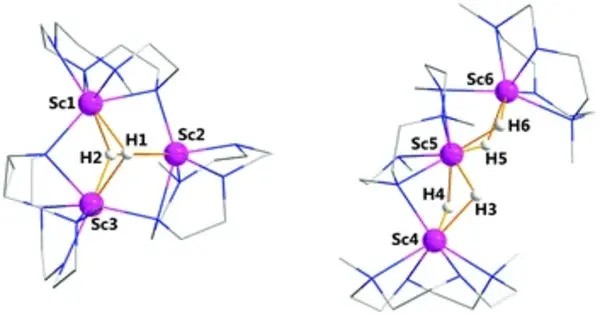
Scandium hydride, also known as scandium–hydrogen alloy, is an alloy made by combining scandium and hydrogen. Hydrogen acts as a hardening agent, preventing dislocations in the scandium atom crystal lattice from sliding past one another. Varying the amount of hydrogen controls qualities such as the hardness of the resulting scandium hydride. Scandium hydride with increased hydrogen content can be made harder than scandium.
It can be formed by progressive hydrogenation of scandium foil with hydrogen. It is a hydride, where scandium is bonded to three hydrogen atoms. The bonding is typically ionic or metallic in nature, depending on the conditions.
Properties
Scandium hydride is a white or colorless solid at room temperature. It can be synthesized by reacting scandium metal with hydrogen gas, often under high pressure and temperature conditions.
ScH₃ is a relatively rare compound, and its properties are not as extensively studied as those of more common hydrides. However, it is known to be a high-energy material and may be used in some advanced materials science and energy storage applications.
Structure
In the narrow range of concentrations which make up scandium hydride, mixtures of hydrogen and scandium can form two different structures. At room temperature, the most stable form of scandium is the hexagonal close-packed (HCP) structure α-scandium. It is a fairly soft metallic material that can dissolve a moderate concentration of hydrogen, no more than 0.89 wt% at 22 °C. If scandium hydride contains more than 0.89% hydrogen at room temperature then it transforms into a face-centred cubic (FCC) structure, the δ-phase. It can dissolve considerably more hydrogen, as much as 4.29%, which reflects the upper hydrogen content of scandium hydride.
Research indicates the existence of a third phase created under extreme conditions termed the η-phase. This phase can dissolve as much as 6.30% hydrogen.
Concentration dependent activation-energies are observed for hydrogen diffusion in scandium metal.
Natural Occurrence
Scandium itself is a rare element, found in trace amounts in the Earth’s crust. It is typically found in ores like thortveitite (Sc2Si2O7) or in small quantities within other minerals like garnet and bauxite. Scandium hydride does not occur naturally on its own in significant quantities because it requires specific conditions (such as hydrogen gas exposure) to form. It is synthesized in laboratories or industrial settings.
Uses
Scandium hydride is of interest in certain niche scientific fields such as hydrogen storage, energy materials, and solid-state chemistry. It may also find use in the development of novel alloys or as a catalyst in certain chemical processes.