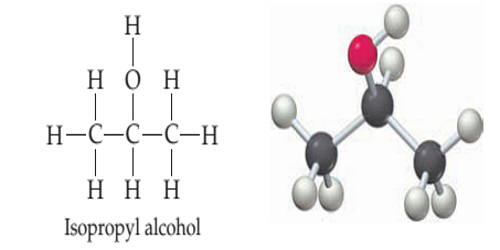
Rubbing alcohol is an isomer of propyl alcohol with antibacterial properties. It is either isopropyl alcohol or ethanol-based liquids, or the comparable British Pharmacopoeia defined surgical spirit, with isopropyl alcohol products being the most widely available. It is a common and surprisingly versatile household item. Rubbing alcohol is undrinkable even if it is ethanol based, due to the bitterants added. It is a cooling and soothing liquid for external application that contains approximately 70 percent denatured ethanol or isopropanol. It is denatured alcohol, typically perfumed, used as an antiseptic or in the massage. It is a liquid that is used to clean wounds or surgical instruments.
They are liquids used primarily as a topical antiseptic. It is a liquid for cleaning medical equipment or a person’s skin so that it is free from bacteria. They also have multiple industrial and household uses. The term “rubbing alcohol” in North American English is a general term for either isopropyl alcohol (isopropanol) or ethyl alcohol (ethanol) products.
All rubbing alcohols are unsafe for human consumption: isopropyl rubbing alcohols do not contain the ethyl alcohol of alcoholic beverages; ethyl rubbing alcohols are based on denatured alcohol, which is a combination of ethyl alcohol and one or more bitter poisons that make the substance toxic. They are various liquid mixtures consisting mainly of either denatured ethyl alcohol or isopropyl alcohol, used to cleanse, cool, or soothe the skin
Properties
All rubbing alcohols are volatile and flammable. Ethyl rubbing alcohol has an extremely bitter taste from additives. The specific gravity of Formula 23-H is between 0.8691 and 0.8771 at 15.56 °C (60.01 °F). It is easily synthesized from the reaction of propylene with sulfuric acid, followed by hydrolysis. It is a colorless liquid having disinfectant properties.
Isopropyl rubbing alcohols contain from 50% to 99% by volume of isopropyl alcohol, the remainder consisting of water. It is used in the manufacture of acetone and its derivatives and as a solvent. Boiling points vary with the proportion of isopropyl alcohol from 80 °C (176 °F) to 83 °C (181 °F); likewise, freezing point varies from −32 °C (−26 °F) to −50 °C (−58 °F).[9] Surgical spirit BP boils at 80 °C (176 °F).
Naturally colorless, products may contain color additives. It is a colorless and flammable liquid, and if you have smelled rubbing alcohol, then you know how isopropyl alcohol smells. They may also contain medically-inactive additives for fragrance, such as wintergreen oil (methyl salicylate), or for other purposes. It is also a volatile liquid, so when its container is left open, it evaporates quickly.