Rhodium (IV) oxide (or rhodium dioxide) is the chemical compound with the formula RhO2. Rhodium, together with platinum, palladium, iridium, ruthenium, and osmium, is one of the platinum- group metals in Group VIII of the Periodic Table. Rhodium metal is a white, hard, ductile, malleable solid with a bluish-gray luster.
Physical Properties
RhO2 has metallic resistivity with values <10−4 Ohm·cm. It transforms in the air to Rh2O3 at 850°C and then to metal and oxygen at 1050°C.
- Compound Formula: RhO2
- Molecular Weight: 134.9
- Appearance: Powder or solid in various forms
- Exact Mass: 134.895333 g/mol
- Monoisotopic Mass: 134.895333 g/mol
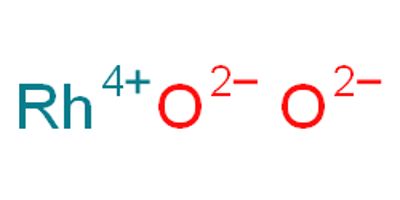
Chemical properties – RhO2 is highly insoluble even in hot aqua regia.
- Form: Powder
- Storage & Sensitivity: Hygroscopic.
- Solubility: It is insoluble in aqua regia.
- Formula Weight: 134.90
Formula weights are especially useful in determining the relative weights of reagents and products in a chemical reaction. These relative weights computed from the chemical equation are sometimes called equation weights.
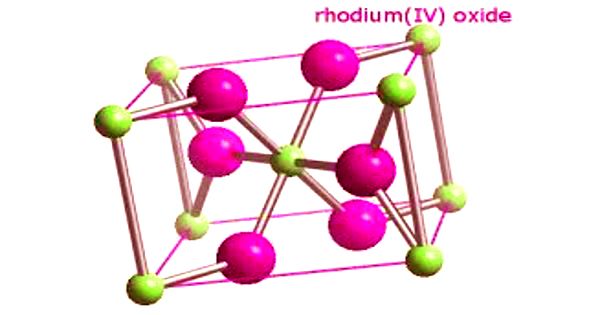
Structure – RhO2 has a tetragonal rutile structure. It is generally immediately available in most volumes.
Applications: Rhodium(IV) oxide has metal anode applications. It has applications for the prediction of drug transport properties. Rhodium has few applications by itself, as in rhodium plating of white gold jewelry or plating of electrical parts, such as commutator slip rings, but, mainly, rhodium is used as a component of platinum alloys. Rhodium-containing catalysts have been proposed for use in automotive catalytic converters for exhaust gas cleanup.