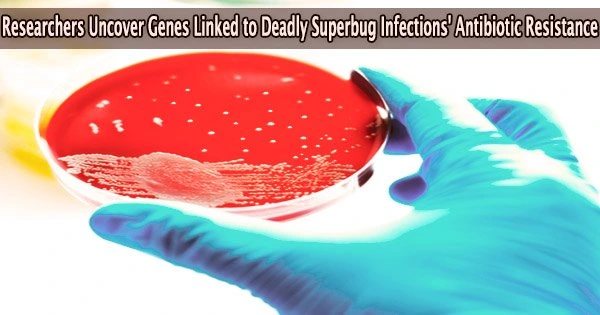
New genetic information about Staphylococcus aureus has been discovered by Australian researchers, shedding light on why the bacterium is so harmful when it enters the blood. Deadly superbug infections, often caused by antibiotic-resistant bacteria, pose a significant threat to public health worldwide.
Superbugs are bacteria that have developed resistance to multiple antibiotics, making them difficult to treat and potentially life-threatening. Despite being frequent, sepsis brought on by Staphylococcus aureus infections, also known as “Golden staph,” can be fatal.
Because of its infamous propensity to develop antibiotic resistance, golden staph is notoriously difficult to treat, which can have negative health effects for people infected with a drug-resistant strain of the bacteria.
Staphylococcus aureus is a Gram-positive bacterium that can be found on the skin and mucous membranes of humans and animals. While it is a part of the normal flora in many individuals, it can also cause a range of infections when it enters the body and multiplies uncontrollably. Staph infections can vary in severity from mild to life-threatening.
In one of the most comprehensive studies of its kind, published in Cell Reports, researchers, led by the Peter Doherty Institute for Infection and Immunity (Doherty Institute), analysed the unique genetic profiles of more than 1,300 Golden staph strains.
The researchers discovered that, while patient characteristics are important in determining mortality risks, specific genes are linked to antibiotic resistance, along with the bacteria’s capacity to persist in the blood and evade the effects of antibiotics and the immune system.
Our study uncovered a deeper understanding of the intricate genetic dynamics underlying severe Golden staph infections. It highlights the potential of combining bacterial whole-genome sequencing, clinical data and sophisticated statistical genomics to discover clinically relevant bacterial factors that influence infection outcomes.
Dr. Stefano Giulieri
University of Melbourne Dr. Stefano Giulieri, a Clinician-Researcher at the Doherty Institute and first author of the paper, said the findings highlighted the diagnostic power of integrating clinical and genomic data.
“To the best of our knowledge, this is one of the first times that the method we used, called a genome-wide association study (GWAS), has been applied to delve into the role of bacterial genomes, host factors and antibiotics on the course of staphylococcal sepsis,” said Dr Giulieri.
“In GWAS, scientists scan the genome of a big collection of bacteria to look for tiny changes (mutations) that show up more often in strains with a certain characteristic, such as antibiotic resistance. Mutations with a strong statistical link are precious clues to figure out how bacteria acquire attributes that are important for patient outcomes.”
“Our study uncovered a deeper understanding of the intricate genetic dynamics underlying severe Golden staph infections. It highlights the potential of combining bacterial whole-genome sequencing, clinical data and sophisticated statistical genomics to discover clinically relevant bacterial factors that influence infection outcomes.”
University of Melbourne Professor Ben Howden, Director of the Microbiological Diagnostic Unit (MDU) Public Health Laboratory at the Doherty Institute and co-senior author of the paper, said that this work represents a significant advancement in medical research as it reshapes our strategies against complex health challenges like Golden staph infections.
“By revealing the genes responsible for antibiotic resistance in Golden staph, our GWAS is pointing the scientific community to clearer targets for the development of effective solutions to treat Golden staph bloodstream infections,” said Professor Howden.
“This knowledge has the potential to shape and enhance our ability to tackle these persistent infections. As bacterial genomes become increasingly available in the clinical routine, we inch closer to customised therapeutic strategies, where treatments will be tailored to the unique genetic makeup of the infecting strain, rather that treating everyone in the same way.”
Deadly superbug infections are a serious public health threat, and addressing this issue requires a coordinated effort from healthcare providers, policymakers, researchers, and the public. Preventing the development and spread of antibiotic-resistant bacteria is essential to safeguarding our ability to treat bacterial infections effectively.
Funding: The National Health and Medical Research Council (NHMRC) and the University of Melbourne funded this research.