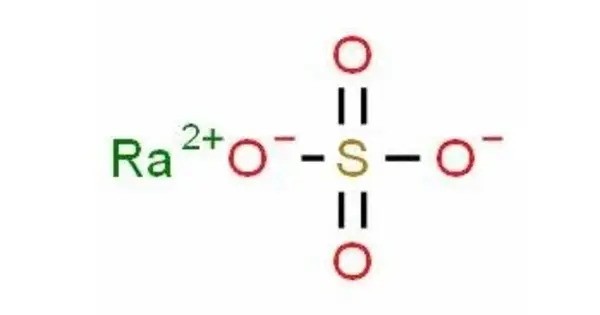
Radium sulfate (or radium sulphate) is an inorganic compound with the formula RaSO4 and an average molecular mass of 322.088 g/mol. It is a white, crystalline solid that is primarily known for being a radioactive compound due to the presence of radium, which is a radioactive alkaline earth metal. This white salt is the least soluble of all known sulfate salts. It was formerly used in radiotherapy and smoke detectors, but this has been phased out in favor of less hazardous alternatives.
Radium sulfate is highly radioactive, as it contains the radioactive isotope radium (Ra). Radium is a member of the actinide series, and it decays into radon gas (Rn), which is also radioactive. It is chemically stable under standard conditions but can react under certain circumstances, particularly in the presence of more reactive agents like acids or alkaline solutions.
Properties
Radium sulfate crystallizes in a solid in the same structure as barium sulfate. It can form solid solutions with the sulfates of strontium, barium or lead. It appears as a white crystalline powder, which is not soluble in water.
- Chemical formula: O4RaS
- Molar mass: 322 g·mol−1
- Appearance: White solid
- Solubility product (Ksp): 3.66×10−11
Formation
Radium sulfate can be synthesized by reacting radium salts with sulfuric acid. It is typically obtained as a byproduct when radium is extracted from ores such as pitchblende (now called pitchblende or uraninite), which also contain uranium.
Natural Occurrence
Radium sulfate can be found in trace amounts in the Earth’s crust due to the natural occurrence of radium. Radium is a decay product of uranium and thorium, both of which are found in minerals such as uraninite and thorite. As radium decays, it forms radium sulfate in the presence of sulfate ions in the environment.
Uses
Due to its radioactivity, radium sulfate has been historically used in medical treatments, such as cancer therapy and in the making of luminous paints. However, these uses have been largely discontinued because of the severe health risks associated with exposure to radium. Radium’s radioactivity can cause serious health effects, including radiation sickness, and is associated with an increased risk of cancer.
Health and Safety Concerns
Radium sulfate, like other radium compounds, poses significant health hazards. Exposure to radium can lead to internal radiation damage, bone cancer, and other illnesses due to its radioactive decay products. Careful handling and proper safety measures are necessary when dealing with radium-containing materials.