Potassium Iodide
Definition
Potassium iodide (KI) is a white crystalline compound used in photography and medicine and as a reagent in chemical analysis. It is also added to table salt to prevent goiter and other iodide-deficiency disorders. Potassium iodide is nontoxic, unless used excessively, but can cause problems for people with iodine sensitivities. In the developing world it is also used to treat skin sporotrichosis and phycomycosis. As a supplement it is used in those who have low intake of iodine in the diet. It is given by mouth.

Potassium Iodide is a metal halide composed of potassium and iodide with thyroid protecting and expectorant properties. Potassium iodide can block absorption of radioactive iodine by the thyroid gland through flooding the thyroid with non-radioactive iodine and preventing intake of radioactive molecules, thereby protecting the thyroid from cancer causing radiation. In addition, this agent acts as an expectorant by increasing secretion of respiratory fluids resulting in decreased mucus viscosity.
It has been used medically since at least 1820. It is on the World Health Organization’s List of Essential Medicines, the most effective and safe medicines needed in a health system. Potassium iodide is available as a generic medication and over the counter. In the United States a course of treatment is less than 25 USD.
Production and Properties of Potassium Iodide
Potassium iodide is made by absorption of iodine in potassium hydroxide:
POTASSIUM IODIDE 7613I2 + 6KOH → 5KI + KIO3 + 3H2O
Most potassium iodate, KIO3, is separated from the product mixture by crystallization and filtration. Remaining iodates are removed by evaporation of the solution and other processes, such as carbon reduction or thermal decompostion at 600ºC to iodide:
2KIO3 → 2KI + 3O2
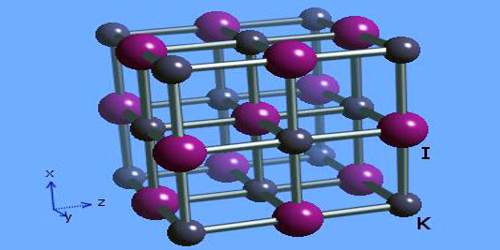
Potassium iodide is ionic, K+I−. It crystallises in the sodium chloride structure. It is produced industrially by treating KOH with iodine.
Another method of preparation that does not involve the formation of iodate is by treating iron turnings with iodine solution. The product, ferrosoferric iodide, Fe3I8•16H2O, is boiled with 15 wt% potassium carbonate solution:
Fe3I8•16H2O + 4K2CO3 → 8 KI + 4CO2 + Fe3O4 + 16H2O
Potassium iodide is Colorless or white cubic crystals or granules; becomes yellowish when exposed to bright light due to photochemical decomposition liberating traces of free iodine; density 3.13 g/cm3; melts at 681°C; vaporizes at 1,330°C; highly soluble in water, ~140 g/100mL at 20°C; aqueous solution readily dissolves iodine; sparingly soluble in ethanol (about 2 g/100mL at 25°C) and acetone; slightly soluble in ether and ammonia.
Uses of Potassium Iodide
Potassium iodide is an inorganic compound that is used as a source of iodine in thyrotoxic crisis and in the preparation of thyrotoxic patients for thyroidectomy. It is added to table salt to make what’s known as iodized salt. Iodized salt typically contains 0.006% to 0.010% potassium iodide.
Potassium iodide (KI) is used with silver nitrate to make silver iodide (AgI) an important chemical in film photography. It is a component in some disinfectants and hair treatment chemicals. KI is also used as a fluorescence quenching agent in biomedical research, an application that takes advantage of collisional quenching of fluorescent substances by the iodide ion. However, for several fluorophores addition of KI in µM-mM concentrations results in increase of fluorescence intensity and iodide acts as fluorescence enhancer.
Potassium iodide is also used to protect people from damage to the thyroid gland caused by radioactive iodine. Radioactive iodine is a byproduct of nuclear fission, and exposure can occur in the presence of nuclear reactors or weapons. It is sometimes used to treat pulmonary diseases that cause a build-up of mucus in the lungs. Potassium iodide works as an expectorant, which means that it loosens this mucus, allowing it to be coughed up more easily.
Reference: