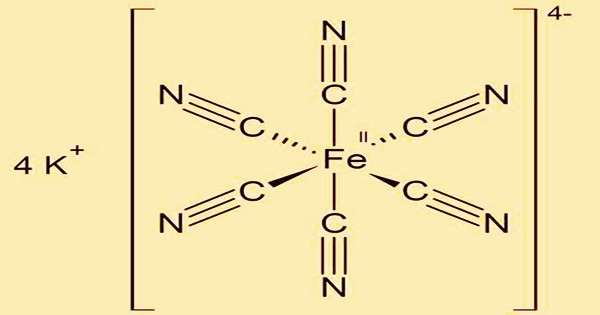
The inorganic compound potassium ferrocyanide, also known as yellow prussiate of potash, has the formula K4(Fe(CN)6)3H2O. It’s a saline-tasting yellow crystal that’s soluble in water, insoluble in alcohol, and loses water at 60°C. It’s used in medicine, dry paints, explosives, and as an analytical reagent, among other things. The coordination complex (Fe(CN)6)4- has a potassium salt. It was made by stirring hot potassium carbonate with an iron rod with wool or horn clippings.
Pierre Joseph Macquer (1718–1784), a French chemist, was the first to mention the preparation of potassium ferrocyanide, which he accomplished by reacting Prussian blue (iron(III) ferrocyanide) with potassium carbonate in 1752. Potassium ferrocyanide was used as a developer and an intermediate for alkaline pyro developers in some iron processes. Dry paints, tempering steel, dyeing, explosives, process engraving and lithography, laboratory reagents, potassium cyanide and ferricyanide.
Chemical compounds containing nitrogen, iron filings, and potassium carbonate were used to make the compound in the past. Torrified horn, leather scrap, offal, and dried blood were all common nitrogen and carbon sources. In the presence of sulphuric acid, potassium ferrocyanide forms potassium sulfate, ferrous sulfate, ammonium sulfate, and carbon monoxide.
K4Fe(CN)6 + 6H2SO4 + 6H2O → 2K2SO4 + FeSO4 + 3(NH4)2SO4 + 6CO
Hydrogen cyanide, ferrous chloride, and calcium hydroxide are used to make potassium ferrocyanide, which is known as Ca2(Fe(CN)6).11H2O in industry. This solution is then treated with potassium salts to produce CaK2(Fe(CN)6), a mixed calcium-potassium salt that is then treated with potassium carbonate to produce the tetrapotassium salt. Iron (III), potassium hexacyanidoferrate(II), and potassium chloride are formed when potassium ferrocyanide reacts with ferric chloride.
K4(Fe(CN)6) + FeCl3 → KFe(Fe(CN)6) + 3 KCl
When heated to red flame, potassium ferrocyanide releases extremely poisonous gases, but the compound itself is not toxic. It is not poisonous to humans because it does not decompose in the body to cyanide. When combined with strong acids, however, this compound can be fatal. H2Fe(NO)(CN)5 is formed when potassium ferrocyanide is treated with nitric acid. Red sodium nitroprusside crystals can be selectively crystallized after neutralization of this intermediate with sodium carbonate.
A 100 g potassium ferrocyanide solution in 1000 ml water must be saturated with chlorine and stirred on a regular basis until it turns a fine, deep red color. In industry, potassium ferrocyanide has a number of uses. It and the sodium salt it is related to are commonly used as anti-caking agents in both road and table salt. In addition to tin purification, potassium and sodium ferrocyanides are used to separate copper from molybdenum ores. Wine and citric acid are also made with potassium ferrocyanide.
In addition to steel tempering, potassium ferrocyanide is used in process engraving. It’s also suitable for animal feed. Potassium ferrocyanide is used in the lab to assess the concentration of potassium permanganate, a compound widely used in redox titrations. Most metal compounds that are insoluble in water form ferro cyanogen, which has highly distinctive colors and can be used to test for cupric and ferric compounds.
Information Sources: