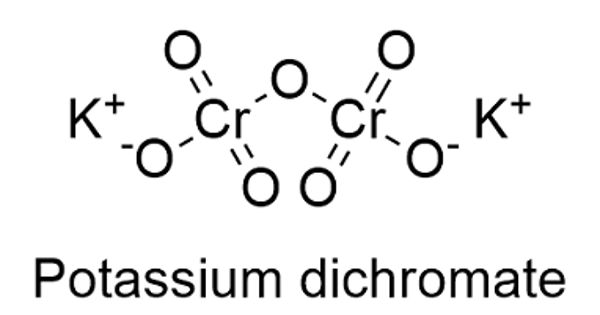
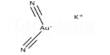
Potassium dichromate, K2Cr2O7, is a potassium salt that is the dipotassium salt of dichromic acid. It is a common inorganic chemical reagent, most commonly used as an oxidizing agent in various laboratory and industrial applications. It is an orange to red-colored, crystalline, inorganic compound that emits toxic chromium fumes upon heating. This substance is used in wood preservatives, in the manufacture of pigments, and in photomechanical processes, but is mainly replaced by sodium dichromate. As with all hexavalent chromium compounds, it is acutely and chronically harmful to health. It primarily affects the respiratory tract causing ulcerations, shortness of breath, bronchitis, pneumonia, and asthma but can also affect the gastrointestinal tract, liver, kidneys, and immune system.
Potassium dichromate is highly corrosive and is a strong oxidizing agent. The salt is popular in the laboratory because it is not deliquescent, in contrast to the more industrially relevant salt sodium dichromate.
Production
Potassium dichromate is usually prepared by the reaction of potassium chloride on sodium dichromate. It is prepared by adding to the neutral yellow chromate of potassium in solution, a moderate quantity of one of the stronger acids. Alternatively, it can be also obtained from potassium chromate by roasting chromite ore with potassium hydroxide. It is soluble in water and in the dissolution process it ionizes:
K2Cr2O7 → 2 K+ + Cr2O72-
Cr2O72- + H2O ⇌ 2 CrO42- + 2 H+

Uses
Potassium dichromate has few major applications, as sodium salt is dominant industrially. It is an important chemical used in industries as an oxidizing agent and for the preparation of many other compounds. The main use is as a precursor to potassium chrome alum, used in leather tanning. It is an important chemical used in industries as an oxidizing agent and for the preparation of many other compounds.
- Cleaning – Like other chromium(VI) compounds (chromium trioxide, sodium dichromate), potassium dichromate has been used to prepare “chromic acid” for cleaning glassware and etching materials.
- Construction – It is used as an ingredient in cement in which retards the setting of the mixture and improves its density and texture. This usage commonly causes contact dermatitis in construction workers.
Natural occurrence
Potassium dichromate occurs naturally as the rare mineral lopezite. It has only been reported as vug fillings in the nitrate deposits of the Atacama Desert of Chile and in the Bushveld igneous complex of South Africa. This substance is a known human carcinogen and is associated with an increased risk of developing lung cancer and cancer of the sinonasal cavity.
Information Source: