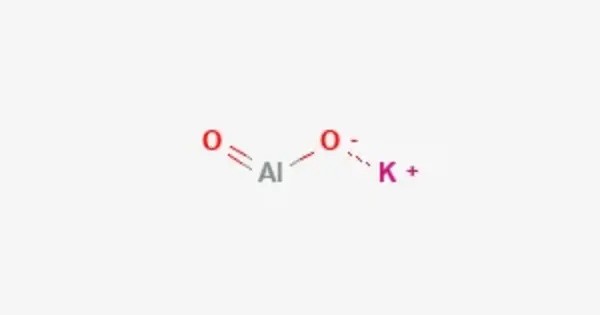
Potassium aluminate is an inorganic compound with the empirical formula KAlO2, which in aqueous solution exists as K[Al(OH)4]. It is a salt that can form in different stoichiometries, including K₄Al₂O₇ and K₂Al₂O₄, depending on the specific reaction conditions. It is typically a white, crystalline solid or powder. It is soluble in water, which makes it useful in a variety of chemical processes. The compound exhibits alkaline characteristics, which can influence the pH of solutions it is present in.
Properties
- Chemical formula: K2Al2O4
- Molar mass: 196.156 g·mol−1
- Solubility in water: very soluble
Reactions
Potassium aluminate can be used to produce potassium alum with sulfuric acid in this reaction.
KAlO2 + 2 H2SO4 ⇌ KAl(SO4)2 + 2 H2O
Production
Synthesis Process: Potassium aluminate can be synthesized by reacting potassium hydroxide (KOH) or potassium carbonate (K₂CO₃) with aluminum oxide (Al₂O₃) at elevated temperatures. This reaction results in the formation of potassium aluminate and the release of heat.
Occurrences
Potassium aluminate is not commonly found in nature but is synthesized through chemical reactions. It is produced by reacting potassium hydroxide (KOH) with aluminum hydroxide (Al(OH)₃) or by reacting aluminum metal with a hot, concentrated solution of potassium hydroxide1.
Applications
- Water Treatment: Used as a coagulant and flocculating agent to purify water.
- Construction: Acts as an accelerating admixture in concrete and cement, especially useful in cold weather.
- Textile Industry: Serves as a mordant to bond dyes to fibers.
- Paper Production: Improves paper quality by strengthening fibers and increasing resistance to heat, light, and mechanical wear.
- Chemical Industry: Functions as a catalyst in various chemical processes.
Safety Considerations
Potassium aluminate is classified as an irritant and can cause skin and eye irritation. It may also induce serious eye damage. It is caustic and can cause irritation to skin and eyes, similar to other alkaline compounds. It should be stored in dry, well-ventilated areas to prevent it from absorbing moisture from the air, which could lead to decomposition or reaction.