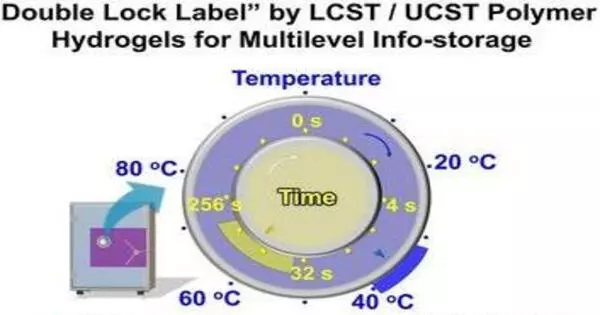
The development of highly secure but simple and inexpensive encryption technology for the prevention of data leaks and forgeries is a significant challenge. A research team published a “double lock” based on thermoresponsive polymer hydrogels in the journal Angewandte Chemie that encrypts information so that it can only be read during a specific temperature and time window.
Physical encryption methods, in addition to digital encryption methods, play an important role. Typically, their decoding is based on external stimuli such as light or heat. Multiple stimuli provide greater security, but make data reading more difficult and complex. “The addition of the time domain greatly increases the chance of achieving the unity of security and simplicity,” according to the Sun Yat-sen University team led by Zhikun Zheng, Xudong Chen, and Wei Liu (Guangzhou, China). “We were inspired by bread baking: delicious bread can only be produced if the baking temperature is neither too low nor too high, and the baking time is neither too short nor too long.”
The addition of the time domain greatly increases the chance of achieving the unity of security and simplicity. We were inspired by bread baking: delicious bread can only be produced if the baking temperature is neither too low nor too high, and the baking time is neither too short nor too long.
Zhikun Zheng
They use thermoresponsive polymer hydrogels – cross-linked chain molecules with water incorporated into their “gaps” – for their novel “double encryption system.” The clear gels become opaque due to partial unmixing when heated above or below a certain temperature. There are two types of gels: LCST and UCST, which have lower or higher critical solution temperatures, respectively. The content of -CO-NH2 groups in the main chain of polymer hydrogels can be used to control phase retention and critical temperature. The rate of the phase transition is determined by the density of cross-linking.
The team used transparent acrylic plates with grooves in the pattern of a QR code as an example of a locked label. Three different gels were placed in specific areas of the pattern: a UCST gel with a phase change around 40 °C and two LCST gels with a phase change around 33 °C (one with a fast phase transition, and one with a slow phase change). The UCST gel is opaque below 20 °C but rapidly shrinks. The pattern is distorted and illegible. It swells between 20 and 33 °C, and a portion of the code formed by this gel becomes readable. The second part of the code, formed by the “fast” LCST gel, remains unreadable.
Only when heated above 33 °C do both LCST gels become opaque. Only the pattern of the “fast” LCST gel has the correct second part of the information now. After about half a minute at 37 °C, it becomes readable and the entire code can be read. However, after only three minutes, the “slow” LCST gel becomes opaque and adds false data, rendering the codes unreadable. At temperatures above 40 °C, both LCST gels become opaque at the same time. Furthermore, the UCST gel becomes opaque and unreadable.
This encryption can be decoded only if the exact temperature and time windows are known. In this example, the source of heat for decoding could be an infrared lamp, a water bath, a hair dryer, or even the human body. These low-cost labels are theoretically suitable for long-term use if sealed to prevent water evaporation.
Because of the world’s rapidly increasing population and industrialization, environmental pollution is a global concern. There is a strong need in this context to develop green separation techniques to isolate pollutants from various sources. Polymer assemblies, also known as hydrogel polymers, are crosslinked polymeric materials that are water-swollen and capable of retaining large amounts of water in their 3D networks.
Because of the presence of various functional groups in their polymeric networks, hydrogels function as excellent adsorbents in pollutant removal processes by binding heavy metal ions and/or organic compounds (e.g., amide, amine, and carboxylic acid). In comparison to other adsorbent materials such as activated carbon, zeolites, silica gel, biomass, and inorganic minerals, hydrogels have the advantages of simplicity, high adsorption, and easy recovery. For these reasons, hydrogels have gained popularity in environmental remediation applications over the last few decades.