Hydrogen bonding refers to the formation of hydrogen bonds, which are a type of attractive intermolecular force caused by the dipole-dipole interaction between a hydrogen atom bonded to a highly electronegative atom and another highly electronegative atom nearby. Hydrogen, for example, is covalently bonded to the more electronegative oxygen atom in water molecules (H2O). As a result of the dipole-dipole interactions between the hydrogen atom of one water molecule and the oxygen atom of another H2O molecule, hydrogen bonding occurs in water molecules.
A research team led by Assoc. Prof. Luo Nengchao and Prof. Wang Feng from the Chinese Academy of Sciences’ Dalian Institute of Chemical Physics (DICP) achieved selective control of the photocatalytic coupling reaction of alcohols. The results of this study were published in the Journal of the American Chemical Society.
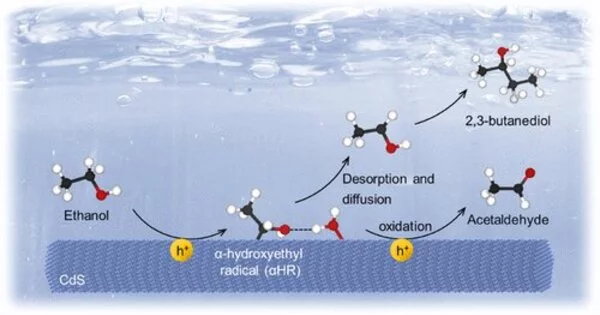
With open-shell electronic structures, radicals are common intermediates in photocatalytic conversions. They are extremely active and readily adsorb on the surface of semiconductors, resulting in a wide range of reactions. The photocatalytic reaction’s product selectivity and quantum yield can be influenced by the solution.
Our study reveals that non-chemical bonding interactions can steer the reaction paths of radicals for selective photocatalysis. With the help of hydrogen bonding, αHR tended to desorb from the catalyst surface and was stabilized in solution, avoiding the predominant oxidation of αHR to acetaldehyde and reverse reaction between αHRs and H· that recovered ethanol.
Luo Nengchao
In this work, the researchers found that adding 5 vol% water to ethanol solution could increase the selectivity of 2,3-butanediol generated by photocatalytic coupling of ethanol from 37% to 57%, with a reaction rate 2.4 times of the original.
Using radical trapping experiments, deuterium experiments, DFT calculations, and molecular dynamics simulations, they found that the introduction of a small amount of water enabled the intermediate α-hydroxyethyl radical (αHR) to form hydrogen bonds with solvent molecules both on the catalyst surface and in bulk solution.
With the help of hydrogen bonding, αHR tended to desorb from the catalyst surface and was stabilized in solution, avoiding the predominant oxidation of αHR to acetaldehyde and reverse reaction between αHRs and H· that recovered ethanol.
“Our study reveals that non-chemical bonding interactions can steer the reaction paths of radicals for selective photocatalysis,” said Luo.
When a hydrogen atom is linked to a highly electronegative atom in a molecule, it attracts the shared pair of electrons more strongly, causing one end of the molecule to become slightly negative while the other end becomes slightly positive. The negative end of one molecule attracts the positive end of the other, resulting in the formation of a weak bond between them. The hydrogen bond is the name given to this type of bond.