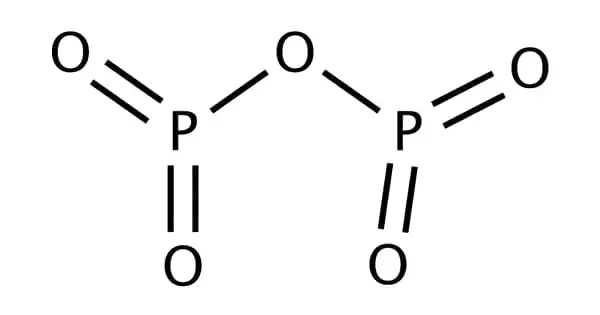
Phosphorous pentoxide is an inorganic chemical compound made up of four phosphorous and ten oxygen atoms. It is commonly found as a dimer of P2O5, which is why it is referred to as phosphorous pentoxide. This white crystalline substance is phosphoric acid anhydride. It is an effective desiccant and dehydrator. It is a white, microcrystalline, light powder formed by the burning of elemental phosphorus in an excess of oxygen.
It is also known as phosphoric anhydride, phosphorous (V) oxide, diphosphorus pentoxide, and other names. It is an acid anhydride derived from phosphoric acid. Because it is highly hygroscopic, it is employed as a dehydration agent and a desiccant.
Properties
Its density is 2.30 g/cm3. It boils at 423 °C under atmospheric pressure; if heated more rapidly it can sublimate. This form can be made by condensing the vapor of phosphorus pentoxide rapidly, and the result is an extremely hygroscopic solid. The reaction with water is very vigorous and proceeds with the evolution of a large amount of heat.
- Molecular Weight: 141.94
- Appearance: White powder
- Melting Point: 580-585 °C
- Boiling Point: 300 °C
- Density: 2.39 g/cm3
- Solubility in H2O: Insoluble

Structure
Phosphorus pentoxide crystallizes in at least four different forms, known as polymorphs. The most common, a metastable form, is made up of P4O10 molecules. These molecules are held together in a hexagonal lattice by weak van der Waals forces. The P4O10 cage structure is similar to adamantane with Td symmetry point group. It is connected to P4O6, the equivalent anhydride of phosphorous acid.
The latter is devoid of terminal oxo groups. The other polymorphs are polymeric, but the phosphorus atoms are connected by a tetrahedron of oxygen atoms, one of which creates a terminal P=O bond by donating terminal oxygen p-orbital electrons to the antibonding phosphorus-oxygen single bonds. The macromolecular form can be created by heating the compound in a sealed tube for several hours and keeping the melt at a high temperature before cooling to solid.
The stable form is a higher density orthorhombic phase known as the O’ form. It is made up of a three-dimensional framework with a density of 3.5 g/cm3. The final polymorph is a glass or amorphous form that can be created by fusing any of the others.
Preparation
P4O10 is prepared by burning tetraphosphorus with a sufficient supply of oxygen:
P4 + 5 O2 → P4O10
For the majority of the twentieth century, phosphorus pentoxide was employed to produce concentrated pure phosphoric acid. The phosphorus pentoxide produced by burning white phosphorus was dissolved in dilute phosphoric acid to make concentrated acid in the thermal process. Because of advancements in filter technology, the “wet phosphoric acid process” is gradually replacing the thermal process, eliminating the requirement to create white phosphorus as a starting material. Phosphoric acid cannot be dehydrated to produce phosphorus pentoxide because when heated, metaphosphoric acid boils without losing all of its water.
Uses
- This chemical is a highly effective dehydrator (since it undergoes hydrolysis in an exothermic manner).
- In several organic synthesis reactions, phosphorus pentoxide is also utilized. It is employed in the synthesis of nitriles from primary amides, for example. It is an essential component of the Onodera reagent, which is frequently used in alcohol oxidation.
- This chemical can also be used to convert mineral acids into anhydrides. For example, N2O5 can be synthesized from HNO3 using P2O5.