Petrochemistry
Definition
Petrochemistry is the branch of chemistry dealing with petroleum or its products. It focuses on how crude oil and natural gas are transformed into raw materials and other useful products. Today, such resources are considered an integral part of the modern economy which makes petrochemistry an incredibly valuable field.
These petrochemicals have become an essential part of the chemical industry today. Major petrochemicals are acetylene, benzene, ethane, ethylene, methane, propane, and hydrogen, from which hundreds of other chemicals are derived.

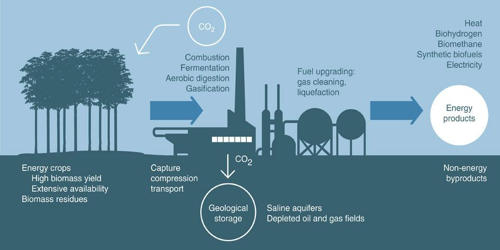
The concentration of organic matter is not very high in the original deposits, but petroleum and natural gas evolved in places that favored retention, such as sealed-off porous sandstones. Petroleum, produced over millions of years by natural changes in organic materials, accumulates beneath the earth’s surface in extremely large quantities. Over millions of years, natural changes in organic materials have produced petroleum which has accumulated under the earth’s surface. Petroleum rich areas are generally found in regions that support retention, such as porous sandstones.
Crude oils are naturally occurring liquids made up of various hydrocarbon compounds that differ in appearance and composition. Average composition rates are 84% carbon, 14% hydrogen, 1%-3% sulphur, and less than 1% each of nitrogen, oxygen, metals and salts. Depending on the sulphur content crude oils are either categorised as sweet or sour.

Petrochemistry and Refining
Industrial filters, technological products and process solutions based on them can be widely used in petrochemistry and refining, being main elements of the following processes:
- Lubricant and oil production
- Desulfurization
- Dehumidification of natural gases
- Catalyst regeneration
- Catalytic reactor
- Fittings and compressor protection
- Crude oil exploitation
- Gas exploitation
Crude oils are compounds that mainly consist of many different hydrocarbon compounds that vary in appearance and composition. Average crude oil composition is 84% carbon, 14% hydrogen, 1%-3% sulphur, and less than 1% each of nitrogen, oxygen, metals and salts. Crude oils with a high sulfur content, which may be in the form hydrogen sulphides, are called sour, and those with less sulphur are called sweet.
Refining is where the job of oil industry stops and that of petrochemical industry starts. The raw materials used in the petrochemistry industry are known as feedstocks. These are obtained from the refinery: naphtha, components of natural gas such as butane, and some of the by-products of oil refining processes, such as ethane and propane.
Petrochemicals go through various processes that eventually contribute to the final output of products like plastics, soaps and detergents, healthcare products like aspirin, synthetic fibres for clothes and furniture, rubbers, paints, insulating materials etc.

Uses of Petrochemistry in Industrial Gases
The most important gas application areas in petrochemistry are identifi ed and illustrated with examples. Requirements on gas production are set forth in terms of quantity, quality and reliability of supply.
Industrial gases are widely applied in petrochemistry, where they are used
- As reactants
- To ensure safety
- To mitigate environmental impacts
- For industrial services
- In analytical procedures
Prominent among gas-using sectors is the chemical industry, where the fi eld of petrochemistry, or petroleum products chemistry, is most important in terms of not only quantities used but also generation of industrial gases.
The most important gases for petrochemistry, apart from light hydrocarbons, are
- The air gases oxygen (O2) and nitrogen (N2)
- Hydrogen (H2)
- Carbon monoxide (CO)
- Synthesis gas or syngas, a mixture of H2 and CO
- Specialty gases such as gas mixtures used for such functions as process or quality control
The global demand for petrochemical products continuously rises. One of the major concerning issues in today’s world is the dependence of the modern society on oil and gas and various other petroleum products. Besides this, there are problems relating to the increasing scarcity of workable hydrocarbon deposits.