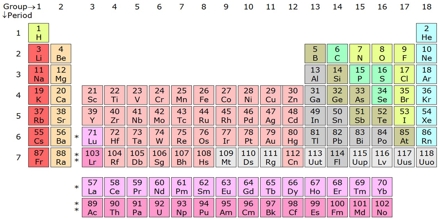
In 1869 the Russian chemist Dmitri Mendeleev and the German chemist J. Lothar Meyer, working independently, found that when the elements were arranged in order of atomic weight they could place them in horizontal rows (one under another), so that the elements in each vertical column had similar properties. This tabular arrangement of the elements in rows and columns, highlighting the regular repetition of properties of the elements is called a periodic table.
The Periodic Table