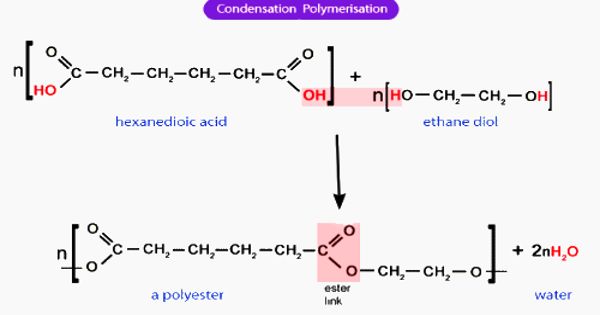
A Penning mixture is a gas combination named after Frans Michel Penning that is utilized in electric lighting or display installations. Although the most common of these is referred to as a neon lamp, it is more effective to fill the glass tube with a Penning mixture, which is described as a mixture of one inert gas with a trace amount of another gas with a lower ionization potential than the main constituent (or constituents).
Explanation
A Penning gas combination is made up of a rare gas with impurity atoms present in very low amounts. The other gas, known as a quench gas, must have a lower ionization potential than the noble gas’s first excited state. The energy of the excited but neutral noble gas atoms can then ionize the quench gas particles via collisions, which is known as the Penning effect.
A relatively common example Some neon lamps, particularly those rated for 120 volts, employ a pening combination of approximately 98–99.5 percent neon and 0.5–2 percent argon. The mixture is easier to ionize than either neon or argon alone, and it reduces the striking voltage at which the tube becomes conductive and begins to emit light. The ideal level of argon is roughly 0.25 percent, but some of it is adsorbed onto the borosilicate glass used in the tubes, thus larger concentrations are needed to account for the losses; higher argon content is used in higher-power tubes since hotter glass adsorbs more argon. The argon alters the color of the “neon light,” turning it slightly yellower. The gas mixtures used in nixie tubes often also included a small amount of mercury vapor, which glowed blue.
Penning transfers, a group of phenomena in which excitation energy is employed to ionize the gas, raise the gas gain in some detectors. The likelihood of such transfers occurring, as well as the process by which they occur, vary with gas composition and pressure. A Penning mixture of neon and argon is also employed as a starting gas in sodium vapor lamps, where it is responsible for the faint reddish glow before the sodium emission begins.
At several hundred torr, the Penning mixture employed in plasma displays is often helium or neon with a tiny proportion of xenon. As filler gases in gaseous ionization detectors, penting mixtures with the formulas argon–xenon, neon–argon, argon–acetylene, and xenon–TMA are utilized. Helium–xenon mixtures are another type of Penning mixture.
Penning ionization and Penning excitation are the processes by which an atom is ionized (or excited) by transferring the excitation energy from a metastable atom whose excitation energy is greater than the ionization (or excitation) energy of the other atom.