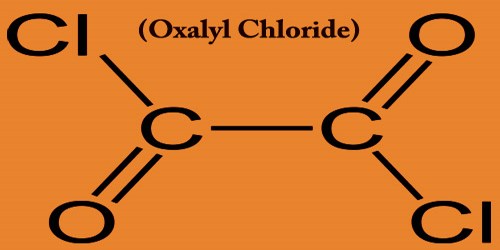
Oxalyl Chloride is a colorless liquid whose formula (COCl)2 has a pungent odor. A valuable reagent in organic synthesis is this colorless, sharp-smelling liquid, the oxalic acid diacyl chloride. Oxalyl chloride is highly poisonous and corrosive. The eyes, skin, and respiratory tract are severely irritated. This can be prepared by taking phosphorus pentachloride to treat oxalic acid.
Oxalyl chloride reacts violently with water; it absolutely was first prepared in 1892 by the French chemist Adrien Fauconnier, who reacted diethyl oxalate with phosphorus pentachloride. Oxalyl chloride is employed within the preparation of cardiolipin analogs which induce peroxidase activity by cytochrome C. It is also used in the preparation of fluorescent indicators for cytosolic calcium.
Oxalyl chloride reacts with gas-only water: hydrogen chloride (HCl), carbon dioxide (CO2), and carbon monoxide (CO).
(COCl)2 + H2O → 2 HCl + CO2 + CO
Oxalyl Chloride reacts vigorously with water, alcohols, and amines and is used for agrochemical, pharmaceutical, and fine chemical synthesis. In this, it’s quite different from other acyl chlorides which hydrolyze with the formation of acid and also the original acid.
The solution containing DMSO and oxalyl chloride, followed by quenching with triethylamine, converts alcohols through the cycle known as the Swern oxidation to the corresponding aldehydes and ketones. Oxalyl Chloride, ClCOCOCl, mol wt 126.9, is produced by the reaction of anhydrous oxalic acid and phosphorus pentachloride. It is primarily used in organic synthesis along with a catalyst of N, N-dimethylformamide to prepare the acyl chlorides from the corresponding carboxylic acids.
Explodes on contact with dimethyl sulfoxide; forms explosive shock-sensitive mixtures with potassium or K-Na alloys. Oxalyl chloride reacts with aromatic compounds within the presence of aluminum chloride to offer the corresponding acyl chloride during a process referred to as a Friedel-Crafts acylation. The resulting acyl chloride is hydrolyzed to make the corresponding acid. Oxalyl chloride was reportedly utilized in the primary synthesis of dioxane tetraketone (C4O6), an oxide of carbon.
Information Sources: