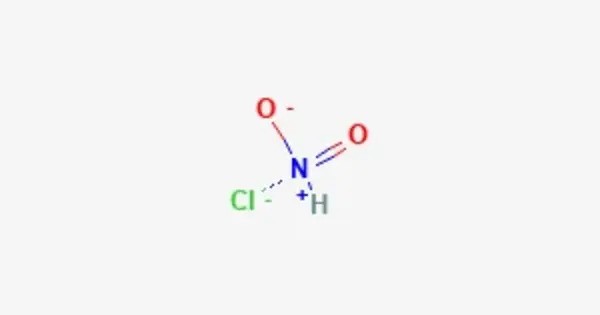
Nitryl chloride is a volatile inorganic compound with formula ClNO2. At standard conditions it is a gas. It is a reactive inorganic compound in which a nitryl group (–NO₂) is covalently bound to a chlorine atom. It is most notable in atmospheric chemistry: formed at night on chloride-containing aerosol surfaces from reactions involving N₂O₅ and chloride, ClNO₂ photolyzes after dawn to release chlorine atoms (Cl·) and NO₂, driving daytime oxidation and influencing urban ozone and particulate formation.
Physically it is a volatile, reactive gas at ambient conditions and must be handled cold and dry. Chemically it is a strong electrophile and oxidant: it hydrolyzes in water to give hydrochloric and nitric acids, reacts with organic matter and many metals, and photolyzes under light. These behaviors make it transient in the environment but important as a nocturnal reservoir of reactive nitrogen and a source of radical chlorine.
Formation
Nitryl chloride can be formed in the reaction of dinitrogen pentoxide with chlorides or hydrogen chloride:
N2O5 + 2HCl → 2ClNO2 + H2O
N2O5 + NaCl → ClNO2 + NaNO3
Properties
- Chemical formula: ClNO2
- Molar mass: 81.46 g·mol−1
- Melting point: −145 °C (−229 °F; 128 K)
- Boiling point: −15 °C (5 °F; 258 K)
- Appearance: Reddish-brown or yellowish gas with a pungent odor
- State: Gas at room temperature
- Density: ~2.74 g/L (at 25 °C, 1 atm)
- Solubility: Hydrolyzes in water, forming nitric acid (HNO₃) and hydrochloric acid (HCl)
- Reactivity: Strong oxidizer; decomposes under UV light or heat; reacts with organic compounds to introduce nitro groups.
- Stability: Unstable and photochemically active, contributing to atmospheric reactions.
Reactions
Nitryl chloride adds to olefins in a radical reaction.
Occurrences
- Atmosphere: Nitryl chloride forms at night in polluted marine and coastal atmospheres when nitrogen oxides (NOx) react with sea salt chloride ions. At sunrise, it photolyzes to release chlorine radicals, which drive tropospheric ozone formation and secondary pollutants.
- Industrial/experimental synthesis: Produced by the reaction of chlorine (Cl₂) with nitrogen dioxide (NO₂).
- Natural occurrence: Rare, but trace amounts may appear in the lower atmosphere due to anthropogenic pollution and marine processes.
Hazards
It is highly reactive, corrosive, and toxic by inhalation; it can cause severe respiratory and eye damage and may promote combustion of organics. Typical controls include low-temperature storage, exclusion of moisture, effective ventilation, and proper respiratory and eye protection.