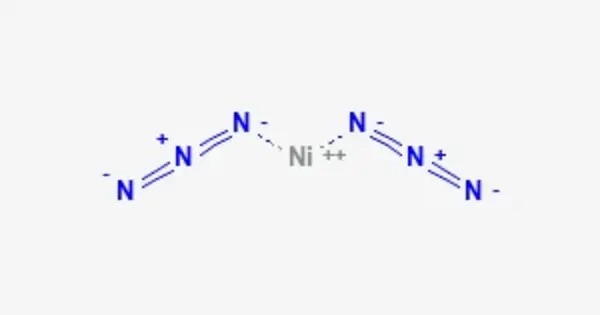
Nickel azide is an inorganic chemical compound with the formula Ni(N3)2. It’s a sensitive and potentially hazardous compound due to the instability of azides, which can decompose explosively under certain conditions, especially when exposed to heat or mechanical shock. It can be formed through the reaction between nickel tetracarbonyl and iodine azide.
2Ni(CO)4 + 2IN3 → Ni(N3)2 + NiI2 + 8CO
Nickel azide is often used in explosives, primarily as a detonator or as part of a larger explosive mixture. Due to its sensitivity and risk, it’s handled with care in specialized environments. The compound is typically synthesized by reacting nickel salts (like nickel nitrate) with sodium azide or other azide compounds.
Properties
Nickel azide water solution has high absorbance in the ultraviolet with a peak at 292 nm. The solution also contains hexaaquanickel cations with visible light absorption peaks at 394, 656, and 720 nm. A related mixed anion compound with nicotinic acid and nicotinate exhibits EO bridging coordination (μ-1,1) on the azide, and possesses an unusual angle between the nickel and nitrogen present within the complex. Like most azides, it is explosive.
- Chemical formula: Ni(N3)2
- Molar mass: 142.73 g/mol
- Appearance: It is typically a yellow-green crystalline solid.
- Chemical Stability: It is highly unstable and sensitive to mechanical shock, friction, and heat. It can decompose explosively under certain conditions.
- Solubility: It is generally insoluble in water, but it can dissolve in some solvents, depending on the specific conditions.
- Decomposition: When it decomposes, it releases nitrogen gas (N₂), which is common for azide compounds, and can potentially cause violent explosions.
- Flammability: It can also be considered hazardous because it is combustible and can burn under certain conditions.
Occurrences
- Laboratory synthesis: Nickel azide is not typically found naturally in the environment. It is synthesized in laboratories, where it is often used in the preparation of explosives, initiators, or in research on materials that involve high-energy reactions.
- Industrial use: It may be found in certain specialized industrial processes or as a part of military materials.
Uses
- Explosives: Nickel azide is sometimes used in explosive mixtures or as a precursor in the synthesis of other azide-based compounds.
- Initiators: It can serve as a detonator or initiator in various explosive devices.
Toxicity
Like other azide compounds, nickel azide is toxic and should be handled with care.