Ion beam milling is a technique used in materials science and microscopy to prepare thin specimens, often referred to as “lamellae,” for analysis under various imaging and analytical tools such as transmission electron microscopes (TEM) or atom probes.
Intracellular biological macromolecules exhibit a more complete and physiological shape as compared to the pure protein in vitro. Therefore, the next objective of structural biology and the frontier of cryo-electron microscopy (cryo-EM) technology development is to directly examine the three-dimensional structure of proteins in vivo.
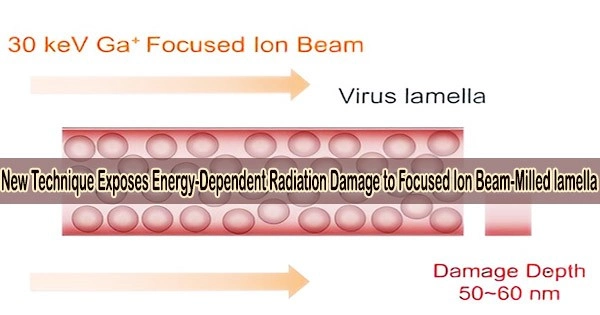
Cells need to be thinned into ~150 nm lamellae to meet the thickness requirement of cryo-EM imaging, and a focused ion beam (FIB) is used to produce the qualified lamellae by milling the frozen cell with high-energy ion beams.
However, FIB destroys the lamellae during milling at both the top and lower surfaces, and it has not yet been possible to conduct a quantitative experimental investigation of the damage or determine how to lessen it.
Researchers from the Institute of Biophysics of the Chinese Academy of Sciences presented a quantitative analysis approach for cryo-FIB damage in a paper that was published in the journal Structure. They also showed that low-energy beam polishing was necessary for creating high-quality thinner lamellae.
This study develops a new technique for calculating the harm that FIB does to cellular lamellae. By using the electron tomography reconstruction, the proteins in cellular lamellae are categorized according to how far they are from the surfaces.
Researchers determined the average signal-to-noise ratio (SNR) of each protein in each group using the 3D reconstruction of the proteins in each group. The average SNRs quantified the degree of FIB damage to the protein at various distances from the surfaces and indicated the data quality of the protein in various groups.
The study discovered that FIB left non-negligible damage on the surface of lamellae at a depth of 50 nm. The damage on the surface was similar to that from exposure to 16 e–/Å2 in a 300-kV cryo-electron microscope and the damage was 3.5 e–/Å2 at ~50 nm region.
The results meant that when the thickness of lamella is less than 100 nm, the damage from the both surfaces will be doubled in the center region, resulting in damage greater than 7 e–/Å2 pre-exposure.
According to theory, the thickness of the cell lamella should be as thin as possible to achieve improved 3D reconstruction resolution. All proteins in the lamella with a thickness of less than 100 nm, however, will be harmed by FIB. It will be challenging to produce the 3D reconstruction for the FIB-milled lamellae with almost atomic resolution due to this disagreement.
In order to break the limitation of the damage depth on the minimum thickness of the lamella, further research found that the damage layer thickness can be reduced to less than 30 nm by reducing the milling voltage of the FIB to 8 keV.
Gaining high-quality lamellae is a crucial prerequisite for enhancing the resolution of 3D reconstruction, which is necessary for the study of protein in situ.
This work not only offered a method for evaluating the lamellae’s quality qualitatively, but it also suggested a plan for minimizing FIB damage in cryo-lamellae, which is helpful for enhancing the resolution of in-situ structure analysis.