Carbon dioxide (CO2) is being converted into raw materials for industrial purposes by research organizations all over the world. The majority of tests under industrially relevant conditions have used heterogeneous electrocatalysts, i.e. catalysts in a different chemical phase than the interacting chemicals. However, homogeneous catalysts in the same phase as the reactants are thought to be more efficient and selective. There have been no setups where homogeneous catalysts could be evaluated under industrial settings to far.
A team headed by Kevinjeorjios Pellumbi and Professor Ulf-Peter Apfel from Ruhr University Bochum and the Fraunhofer Institute for Environmental, Safety and Energy Technology UMSICHT in Oberhausen has now closed this gap. The researchers outlined their findings in the journal Cell Reports Physical Science.
“Our work aims to push the boundaries of technology in order to establish an efficient solution for CO2 conversion that will transform the climate-damaging gas into a useful resource,” says Ulf-Peter Apfel. His group collaborated with the team led by Professor Wolfgang Schöfberger from the Johannes Kepler University Linz and researchers from the Fritz Haber Institute in Berlin.
Our findings open up the possibility of testing and integrating high-performance and easily variable homogeneous electrocatalysts in application scenarios for electrochemical processes.
Ulf-Peter Apfel
Efficiency and long-lasting stability
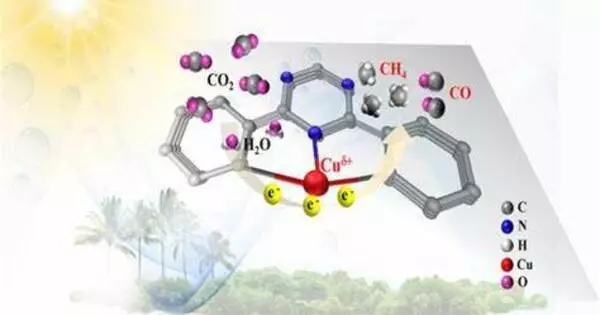
The researchers investigated CO2 conversion via electrocatalysis. A voltage source gives electrical energy to the reaction system via electrodes, which causes the chemical transformations at the electrodes. The reaction is aided by a catalyst, which is often a dissolved metal complex in homogeneous electrocatalysis. The initial material CO2 passes past the electrode in a gas diffusion electrode, where the catalysts convert it to carbon monoxide. In turn, the latter is a frequent starting material in the chemical industry.
The metal complex catalysts were incorporated into the electrode surface without being chemically bonded to it. They demonstrated that their technology was capable of efficiently converting CO2: It produced current densities in excess of 300 milliamperes per square centimeter. Furthermore, the system remained stable for almost 100 hours with no evidence of deterioration.
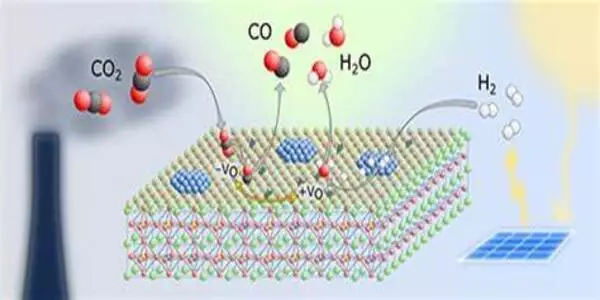
No need to anchor the catalyst
Developing novel catalyst systems for CO2 conversion is a crucial area of research aimed at addressing climate change and reducing greenhouse gas emissions. Several approaches are being explored to efficiently convert carbon dioxide (CO2) into valuable products, such as fuels or chemicals.
All of this indicates that homogeneous catalysts can be employed in electrolysis cells in general. “However, they do require a specific electrode composition,” Ulf-Peter Apfel emphasizes. More particular, the electrodes must allow direct gas conversion without the use of solvents in order to prevent the catalyst from being leached from the electrode surface. There is no need for a carrier substance that chemically binds the catalyst to the electrode surface, contrary to what is commonly described in expert literature.
“Our findings open up the possibility of testing and integrating high-performance and easily variable homogeneous electrocatalysts in application scenarios for electrochemical processes,” Apfel says in a statement.