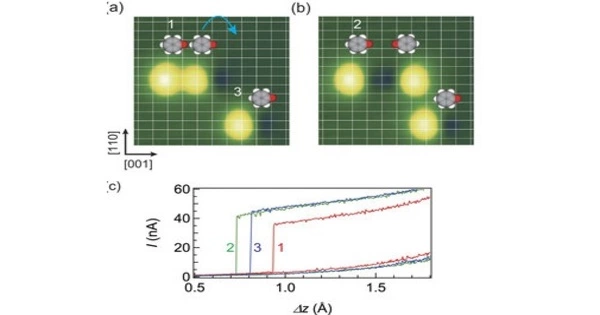
Molecular conductance (G=I/V), often known as single-molecule conductance, is a physical quantity in molecular electronics. It refers to a molecule’s ability to carry electrical current. It is a property that is determined by the molecule’s molecular structure and electronic characteristics. It is a key notion in molecular electronics, which investigates the use of molecules as functional components in electronic systems.
Molecular conductance is affected by environmental factors such as pH, temperature, and pressure, as well as the parameters of the measurement apparatus. Many experimental procedures have been devised in an attempt to directly quantify this number, but theorists and experimenters continue to confront numerous hurdles. A molecule’s conductance can be explained using its electronic structure and the energy levels of its molecular orbitals. When a voltage is placed across a molecular junction, electrons in the molecule can travel from one electrode to another via the molecular bridge. The molecular conductance is determined by the ease with which these electrons can move or the resistance they meet.
Several factors influence molecular conductance:
- Molecular Structure: The arrangement of atoms in a molecule is critical. Because they allow for better electron delocalization, molecules with conjugated systems (alternating single and double bonds) have higher conductance.
- Energy Levels: Conductance is also affected by the alignment of energy levels, such as the highest occupied molecular orbital (HOMO) and the lowest unoccupied molecular orbital (LUMO), with the electrode’s Fermi level. Electron transfer is more efficient when these energy levels are well-matched.
- Anchoring Groups: Anchoring groups that produce strong chemical connections are frequently used to bind molecules to electrodes. The anchoring groups used can impact the electrical connection between the molecule and the electrode, which affects conductance.
- Length of the Molecular Bridge: Longer molecules generally have higher resistance and lower conductance than shorter ones because electrons encounter more obstacles when traversing a longer path.
- Temperature: Conductance can also depend on temperature. Some molecular junctions may exhibit temperature-dependent conductance due to changes in molecular conformation or other factors.
A significant amount of progress has recently been achieved in the development of dependable conductance-measuring techniques. These approaches are classified as molecular film experiments, which measure groups of tens of molecules, and single-molecule measurements.
Molecular electronics researchers strive to build and study molecules with certain conductivity qualities in order to create molecular-scale electrical devices such as molecular wires, switches, and diodes. Understanding molecular conductance is critical for moving forward with the creation of nanoscale electronic components and perhaps transforming the area of electronics.