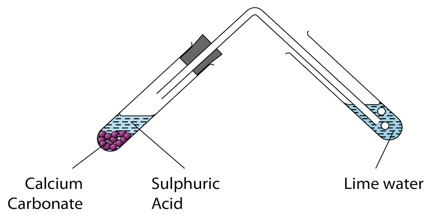
Prime purpose of this lecture is to present on Metals and Acids. The products of acid/metal reactions are a salt and hydrogen gas. Some metals are so unreactive that they do not react with dilute acids at all, e.g. copper, silver and gold. Examples of Metals: Magnesium, Iron, Sodium, Calcium etc. Examples of Acids: Hydrochloric acid; Sulphuric acid; Nitric acid; Ethanoic acid etc. The chemical reaction of Metals and Acids: Metal + Acid → Salt + Hydrogen.
Metals and Acids