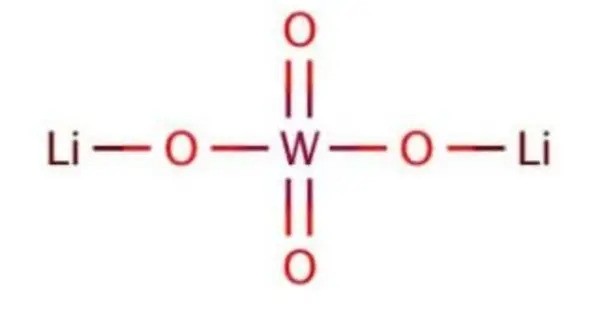
Lithium tungstate is the inorganic compound with the formula Li2WO4. It is a white solid that is soluble in water. It is a solid material with a crystalline structure that exhibits a variety of interesting properties. The compound is one of the several orthotungstates, compounds that feature the tetrahedral WO42− anion. It is a notable material because of its unique properties, making it useful in various high-tech applications, including electronics and optics.
Properties
Lithium Tungstate is typically a white crystalline solid, although it can also form in a powder form. It is sparingly soluble in water, but its solubility can vary depending on temperature and conditions. It has semiconducting properties, making it useful in certain electronic and optical applications.
- Chemical formula: Li2WO4
- Appearance: white solid
- Density: 4.56 g/cm3
- Formation: It is commonly synthesized by reacting tungsten oxides (such as WO₃) with lithium compounds (like Li₂CO₃ or LiOH).
- Reactivity: It is relatively stable at room temperature, but it can react with acids and bases under specific conditions.
- Solubility: It is sparingly soluble in water and has low solubility in organic solvents.
Structure
The salt consists of tetrahedrally coordinated Li and W centres bridged by oxides. The W-O and Li-O bond distances are 1.79 and 1.96 Å, respectively. These differing bond lengths reflect the multiple bond character of the W-O interaction and the weaker ionic bonding between the Li-O interactions. The solid undergoes phase transitions at high pressures, such that the coordination geometry at tungsten becomes octahedral (six W-O bonds). For example at 40 kilobars, it adopts a structure related to wolframite.
Occurrences
- Natural Occurrence: Lithium tungstate is rarely found in nature in its pure form. Tungsten, however, is commonly found in minerals like scheelite (CaWO₄) and wolframite ((Fe,Mn)WO₄), which are the primary sources of tungsten. Lithium, on the other hand, is most commonly found in minerals like spodumene (LiAlSi₂O₆), lepidolite (LiAlSi₃O₁₀(OH)₂), and petalite (LiAlSi₄O₁₀).
- Synthesis: Lithium tungstate is generally synthesized by the reaction of lithium salts (such as lithium carbonate) with tungsten oxide (WO₃) at high temperatures. This method allows for the production of the compound in a controlled environment.
- Industrial Occurrence: Lithium tungstate may be produced as part of specialized industrial applications that require materials with high melting points, electrical conductivity, or optical properties. This includes areas like laser technology, electronics, and certain high-temperature processes.
Uses
Lithium tungstate is used to produce high density aqueous polytungstate (metatungstate) solutions. Like other high density fluids, such solutions are often used in the separation of minerals and other solids. These can achieve a density of 2.95 at 25 °C and up to 3.6 in hot water.
- Materials Science: It has been explored for use in solid-state electrolytes, optical materials, and in some high-temperature applications.
- Ceramics: It is used in ceramic compositions, especially in the preparation of specific tungsten-based materials.
- Neutron Absorption: Due to its ability to interact with neutrons, lithium tungstate has been investigated for use in nuclear reactors or radiation shielding.
- Catalysis: It can serve as a catalyst or catalyst support in certain chemical reactions, especially in the context of organic synthesis.