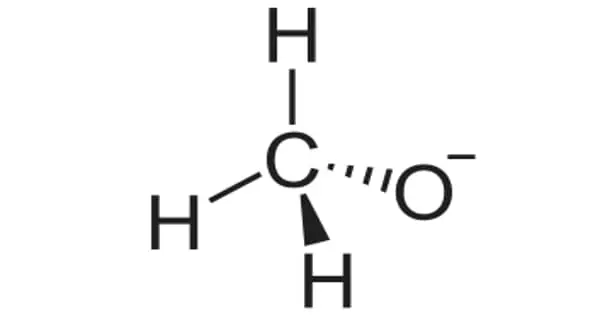
The formula for lithium methoxide is LiCH3O. It is methanol’s lithium salt. Unlike the lithium alkoxides derived from heavier alcohols, lithium methoxide’s bonding is predominantly ionic. It is insoluble in common polar aprotic solvents such as THF; however, it is soluble in methanol and is commercially available as a 10% solution.
Methoxides are organic salts that are the most basic alkoxides. Other metal-cation variants such as lithium methoxide, rubidium methoxide, and caesium methoxide exist in addition to sodium and potassium methoxide.
Properties
- Melting point: 500°C
- Boiling point: 64.6 °C
- Density: 0.85 g/mL at 20 °C
- Flash point: 52 °F
- Storage temp.: Flammables area
- Solubility: Soluble in methanol.
- Form: powder
- Color: White
- Specific Gravity: 0.85

Preparation and Standardization of 0.1 M Lithium Methoxide
Lithium Methoxide Solution Preparation –
- Dissolve in small portions 0.7 g of freshly cut lithium in 150 ml of anhydrous methanol, cooling the flask during the addition of the metal.
- When the reaction is complete add sufficient toluene to produce 1000 ml.
- If cloudiness or precipitation occurs, add sufficient anhydrous methanol to clarify the solution.
- Standardise the solution immediately before use in the following manner.
Lithium Methoxide Solution Standardization –
- Weigh accurately about 0.25 g of benzoic acid, dissolved in 25 ml of dimethylformamide.
- Titrate with lithium methoxide solution, using quinaldine red solution as the indicator and protecting the solution from atmospheric carbon dioxide throughout the titration.
- Perform a blank determination and make any necessary correction.
- 1 ml of 0.1 M lithium methoxide is equivalent to 0.01221 g of C7H602.
Purification Methods
LiOH is the most likely impurity as a result of moisture hydrolysis. It’s critical to keep the sample dry. It can be dried in a vacuum at 60-800 under dry N2 using an oil pump for a few hours. In the cold, store it under N2.