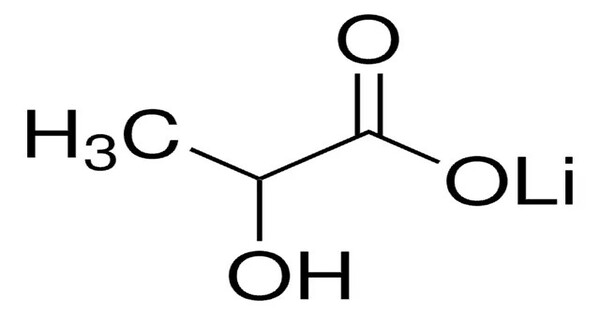
Lithium lactate is a chemical compound, a salt of lithium and lactic acid with the formula CH3CH(OH)COOLi, an amorphous solid, very soluble in water. It is a compound that combines lithium, a naturally occurring alkali metal, with lactate, the ion form of lactic acid. It is primarily used in medical and pharmaceutical applications.
Lithium lactate has been historically used as a mood stabilizer in the treatment of bipolar disorder. Lithium salts, including lithium lactate, are effective in reducing the frequency and severity of manic episodes in bipolar patients.
Properties
- Chemical formula: C3H5LiO3
- Molar mass: 96.01
- Appearance: Amorphous solid
- Melting point: 300 °C (572 °F; 573 K)
- Solubility in water: Very soluble
Synthesis
Synthesis is by neutralization of lactic acid with lithium hydroxide:
LiOH + CH3CH(OH)COOH → CH3CH(OH)COOLi + H2O
Physical properties
- Lithium lactate forms an amorphous solid.
- It dissolves very well in water and organic solvents.
- The compound demonstrates optical isomerism.
- Lithium lactate emits acrid smoke when heated to decomposition.
Chemical properties
Lithium lactate is a white, crystalline solid that is highly soluble in water. Its chemical formula is C3H5LiO3. It reacts with triphosgene to obtain lactic acid-O-internal anhydride. It can be used as a precursor to prepare Li4SiO4, Li4Ti5O12/C and other materials.
Use
Lithium lactate is part of drugs that promote the excretion of uric acid from the body.
It is also used as an Antipsychotic.
Side Effects
Like all medications, lithium lactate can cause side effects. Common side effects include nausea, diarrhea, dizziness, and drowsiness. More serious side effects can occur at higher doses, including tremors, confusion, and kidney problems.