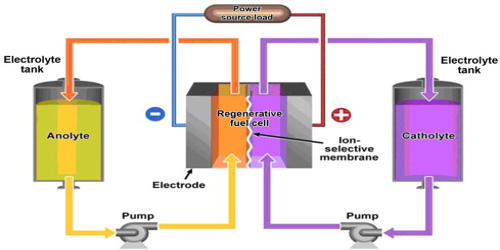
At low temperatures, liquid nitrogen [LN2] is nitrogen in a liquid state. It is a cryogenic liquid that is extensively utilized in research laboratories. The boiling point of liquid nitrogen is approximately 195.8 °C (320 °F; 77 K). It can expand to a very big amount of gas when liquefied under high pressure conditions. It is manufactured industrially using fractional distillation of liquid air. It is a colorless liquid with a low viscosity that is commonly used as a coolant. is a type of nitrogen that is cold enough to exist as a liquid and is employed in a variety of cooling and cryogenic applications.
The liquefied form of the element nitrogen generated commercially by fractional distillation of liquid air is known as liquid nitrogen. It is made up of two nitrogen atoms that share covalent bonds, just like nitrogen gas (N2).
Physical properties
Liquid nitrogen is colorless, odorless, inert, noncorrosive, nonflammable, and extremely cold. The majority of the atmosphere is composed of nitrogen (78 percent by volume). Nitrogen is inert and does not support combustion; however, it does not support life. Nitrogen becomes a cryogenic liquid when it is transformed to liquid form.
After liquefaction, the N2 molecule retains its diatomic nature. Because of the weak van der Waals connection between the N2 molecules, there is little interatomic interaction, resulting in a relatively low boiling point.

By placing liquid nitrogen in a vacuum chamber pumped by a vacuum pump, its temperature may be easily decreased to the freezing point of 210 °C (346 °F; 63 K). The efficacy of liquid nitrogen as a coolant is restricted by the fact that it boils instantly when it comes into contact with a warmer item, engulfing the object in an insulating layer of nitrogen gas bubbles. The Leidenfrost effect occurs when a liquid comes into contact with a surface that is much hotter than its boiling point. It is possible to achieve faster cooling by immersing an object in a slurry of liquid and solid nitrogen rather than just liquid nitrogen.
To prevent pressure buildup, liquid nitrogen is stored in special insulated containers that are vented. Depending on the design of the Dewar flask, it can be stored for a few hours or for several weeks.
Application
Liquid nitrogen, with a boiling point of -196 degrees Celsius, is used for a variety of purposes, including computer cooling, medicine to remove undesirable skin, warts, and pre-cancerous cells, and cryogenics, which studies the effect of extremely cold temperatures on materials. It’s also becoming more popular in high-end restaurants as a way to immediately freeze food and drinks or create a spectacular cloud of vapour or fog when exposed to air.
Because of its low reactivity and cold temperature, liquid nitrogen has a wide range of applications. The following are some examples of common applications:
- The freezing and transporting of food products,
- It is use as a coolant for superconductors, vacuum pumps, and other materials and equipment,
- It is use in cryotherapy to remove skin abnormalities,
- A source of extremely dry nitrogen gas,
- The branding of cattle.
- The molecular gastronomy preparation of unusual foods and beverages.
Hazards
Even brief exposure to liquid nitrogen vapor can cause fast freezing of skin tissue and ocular fluid, resulting in cold burns, frostbite, and permanent eye damage.
Oxygen condensed from the air may collect in liquid nitrogen tanks. There is a risk of severe oxidation of organic materials as the nitrogen evaporates.