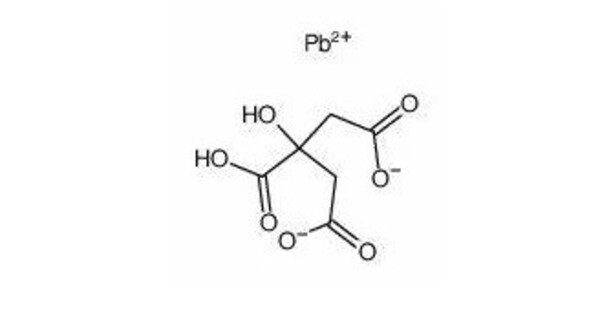
Lead citrate is a compound of lead and citrate that is primarily used as an enhancer for heavy metal staining in electron microscopy. It serves as a stain to enhance contrast in specimens, allowing for better visualization of cellular structures. This salt binds to osmium and uranyl acetate and enhances contrast in many cellular structures. Lead citrate is highly reactive with carbon dioxide. It is often used in biological and biochemical applications, particularly in electron microscopy.
When applied, lead citrate reacts with cellular components, helping to outline organelles and membranes. The compound tends to be more soluble at lower pH levels due to the protonation of the citrate anion.
Properties
It typically appears as a yellowish or white powder. It is moderately soluble in water and more soluble in acidic conditions. It can also dissolve in some organic solvents. It is generally stable under standard conditions but can decompose when exposed to heat or strong acids.
- Chemical formula: C12H10O14Pb3
- Molar mass: 999.8 g·mol−1
- Appearance: White odorless powder or crystals
- Density: 4.63 g/cm3
- Boiling point: 309.6 °C (589.3 °F; 582.8 K)
- Solubility in water: Soluble in water, slightly soluble in alcohol
Occurrences
- Biological Systems: Lead citrate can form in biological systems where lead is present, often as a result of contamination. Its occurrence in tissues can be relevant in studies of lead toxicity.
- Histological Staining: It is frequently used as a staining agent in electron microscopy to enhance contrast in biological samples, particularly for visualizing cellular structures.
- Chemical Research: In research settings, lead citrate can be synthesized for specific applications in analytical chemistry and materials science.
Safety Considerations
Lead citrate contains lead, a toxic heavy metal, so it should be handled with care, following appropriate safety protocols to avoid exposure and contamination.