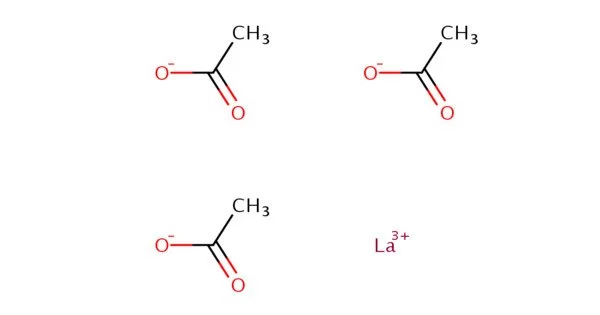
Lanthanum acetate is an inorganic compound, a salt of lanthanum with acetic acid with the chemical formula La(CH3COO)3. It is a chemical compound composed of lanthanum, a rare earth element, and acetate, a negatively charged ion derived from acetic acid. It is used in various chemical and research applications, particularly in processes related to lanthanum chemistry. Lanthanum itself has applications in catalysts, phosphors, and as a component in certain electronic devices.
Lanthanum is a member of the lanthanide series, a group of elements that includes elements with atomic numbers from 57 to 71. Lanthanides are known for their similar chemical properties, and they are often collectively referred to as rare earth elements. Acetate is the ion derived from acetic acid, a weak organic acid. Acetate ions are commonly found in various chemical compounds, and they can form salts with metals, including lanthanum acetate.
Synthesis
Lanthanum acetate can be formed by the reaction of lanthanum(III) oxide and acetic anhydride:
La2O3 + 3(CH3CO)2O → 2La(CH3COO)3
It is also made in a reaction of lanthanum oxide with 50% acetic acid:
La2O3 + 6CH3COOH → 2La(CH3COO)3 + 3H2O
Properties
- Appearance: It is usually found as a white or colorless crystalline powder.
- Solubility: It is typically soluble in water. The solubility may vary depending on the specific hydrate or form of the compound.
- Hydrates: It may exist in different hydrate forms, indicating that a certain number of water molecules are associated with each formula unit of the compound.
- Formula Weight: The molar mass or formula weight of lanthanum acetate depends on the specific hydrate form and is calculated by adding the atomic masses of lanthanum, carbon, hydrogen, and oxygen in the compound.
Uses
Lanthanum acetate is used in specialty glass manufacturing and in water treatment. Also, it is used to produce porous lanthanum oxyfluoride (LaOF) films. It is also used as a component in the production of ceramic products and as a catalyst in the pharmaceutical industry.