The body is constantly in motion. Your skin’s cells, in particular, are constantly replacing themselves. The skin accomplishes this through the regeneration and repair processes. Skin cells shed constantly on a cellular level, revealing fresh, newly grown skin cells beneath. This is why scars and blemishes fade over time.
Throughout our lives, the epidermis, or outer layer of skin, is constantly turning over to replace dead or damaged cells. Skin stem cells must constantly decide whether to make more copies of themselves (self-renewal) or to change their fate to differentiation. The molecular mechanisms governing this process are still unknown. Now a research team has identified a molecular switch that plays an early and critical role in the skin stem cell differentiation process.
It’s sunburn season. Many of us have felt the pain and peeling caused by unprotected sun exposure, but we may overlook a remarkable and vital part of the process: the regeneration of skin as damaged tissue is replaced with new.
Even when we are not exposed to the sun, the epidermis, or outer layer of skin, is constantly turning over to replace dead or damaged cells. This epidermal layer serves as an important barrier for the human body, reducing water loss and combating environmental threats. Scientists are working to identify the molecular mechanisms that control skin epidermal regeneration, but much remains unknown.
Skin stem cells must constantly decide whether to make more copies of themselves or to change their fate to differentiation. A delicate balance between these two decisions is required to maintain skin integrity and barrier function.
Xiaomin Bao
Now a Northwestern University research team has identified a molecular switch, through a protein called CDK9, that plays an early and critical role in the skin stem cell differentiation process. This switch is “off” in the stem cells. When the switch is turned on, a specific group of genes is immediately activated to trigger downstream gene regulators, allowing the skin cells to progressively gain barrier function. The findings have relevance for improved understanding of cancer and wound healing, in addition to the fundamental understanding of skin regeneration.
“Skin stem cells must constantly decide whether to make more copies of themselves (a process known as self-renewal) or to change their fate to differentiation. A delicate balance between these two decisions is required to maintain skin integrity and barrier function” Xiaomin Bao, a stem cell biologist at Northwestern who oversaw the study, said “We discovered the switch bound to specific genomic regions within stem cells, ready to activate the cell fate switch, initiating the stem cell’s movement towards differentiation.”
Bao is an assistant professor of molecular biosciences in the Weinberg College of Arts and Sciences and an assistant professor of dermatology at the Feinberg School of Medicine at Northwestern University. Her lab studies the fundamental biology of the process of skin stem cell differentiation.
The study was published recently by the journal Nature Communications.
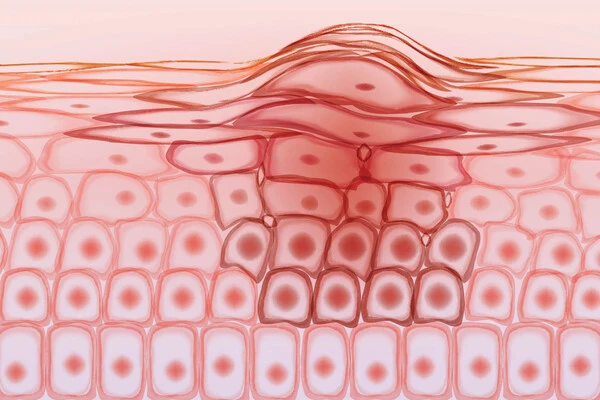
Discovery of the switch
The integrity of skin epidermis relies on subsets of skin stem cells to continuously self-renew or differentiate, compensating for daily wear and tear. The differentiation process involves significant changes from more than 6,000 genes, ceasing stem cell proliferation while activating barrier-function genes.
Bao and her colleagues discovered that the kinase activity switch of the protein CDK9 plays a key role in the decision of cells to initiate differentiation and progressively acquire the barrier function of the tissue by integrating genomics, genetics, and pharmacological inhibition in human skin models. In the stem cell state, kinase activity is inhibited, and the rapid-response genes directly controlled by the kinase are suppressed. When the kinase activity is turned on, the rapid-response genes are activated, which then activates the downstream effectors, which are a group of transcription factors that can further drive the expression of barrier-function genes.
CDK9 (cyclin-dependent kinase 9) plays crucial roles in modulating gene expression at the step of “transcription,” a process of copying specific DNA regions to RNA, before RNA can serve as templates for synthesizing new proteins. In the stem cell state, CDK9 is maintained in the “off” state when bound together with the proteins AFF1 and HEXIM1 on DNA, awaiting specific cellular signals such as the activation of protein kinase C signaling. Once the signaling is activated, this is sufficient to switch CDK9 from the inactive to the active state, allowing the rapid synthesis of RNA from the genomic regions directly bound by CDK9, the researchers found.
The transition is quick. “All of the components are ready to go deep inside the stem cells,” said Bao. When a stem cell receives a specific external signal, the response inside the nucleus is very fast, with activated CDK9 causing rapid-response genes like ATF3 to be expressed in as little as one hour. ATF3 expression strongly stimulates the expression of several downstream transcription factors, rewiring the cell fate toward differentiation. This quick switch for gene activation is also based on the recruitment of RNA-synthesis machinery and CDK9 to rapid-response genes prior to signaling activation.
“We are probing the unknown,” Bao said. “Stem cell regulation is fundamental for sustaining the integrity of human tissue. We have found a key mechanism initiating the fate switch of skin stem cell towards differentiation, an integral process of regeneration. Learning more about the fundamental molecular mechanisms can help in the understanding of many different human diseases.”