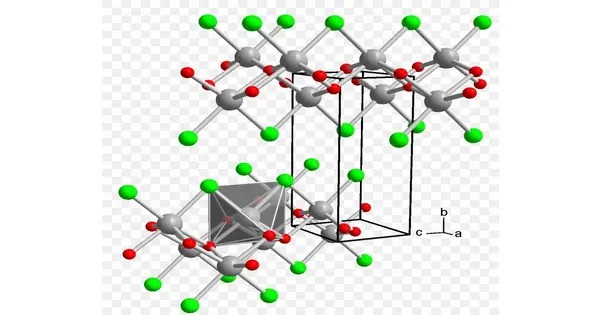
Iron oxychloride is the inorganic compound with the formula FeOCl. It’s an inorganic solid and is best known as part of a family of materials known as iron oxyhalides. This purple solid adopts a layered structure, akin to that of cadmium chloride. The material slowly hydrolyses in moist air. The solid intercalates electron donors such as tetrathiafulvalene and even pyridine to give mixed valence charge-transfer salts. Intercalation is accompanied by a marked increase in electrical conductivity and a color change to black.
Properties
- Chemical formula: ClFeO
- Molar mass: 107.29 g·mol−1
- Appearance: Vivid, dark violet, opaque crystals
- Solubility: Slowly hydrolyzes in water
- Stability: Sensitive to moisture and decomposes in water, forming iron(III) hydroxide and hydrochloric acid
Production
FeOCl is prepared by heating iron(III) oxide with ferric chloride at 370 °C (698 °F) over the course of several days:
Fe2O3 + FeCl3 → 3 FeOCl
Alternatively, FeOCl may be prepared by the thermal decomposition of FeCl3⋅6H2O at 220 °C (428 °F) over the course of one hour:
FeCl3 ⋅ 6H2O → FeOCl + 5 H2O + 2 HCl
Synthesis
FeOCl can be synthesized by heating iron(III) chloride (FeCl₃) in the presence of water vapor or oxygen at high temperatures:
FeCl₃ + H₂O (g) → FeOCl + 2HCl
Chemical and Structural Feature
FeOCl has a layered structure, where Fe³⁺ ions are coordinated by oxygen and chlorine atoms. These layers are held together by van der Waals forces, making it a candidate for intercalation reactions (similar to graphite or MoS₂). The iron is typically in the +3 oxidation state.
Natural Occurrence
Found as a rare mineral called minasragrite (sometimes FeOCl is referred to as an alteration product in iron sulfide environments). Not common in nature — usually occurs in oxidizing zones of iron sulfide deposits.
Applications
- Catalysis: Used as a catalyst or catalyst precursor in organic reactions
- Research: Studied for intercalation chemistry (layered compounds can incorporate ions or molecules between their layers)
- Materials Science: Interest in electronic and magnetic properties