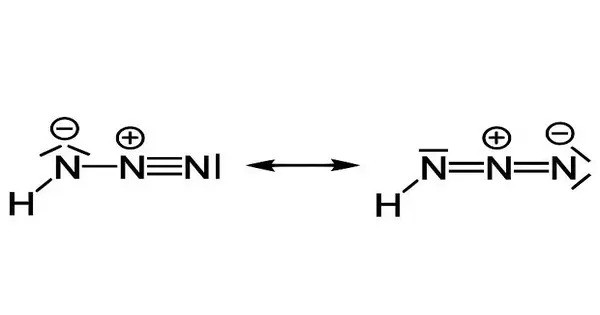
Hydrazoic acid, also known as hydrogen azide, azic acid or azoimide, is a compound with the chemical formula HN3. It is a highly reactive and toxic compound containing nitrogen, hydrogen, and azide groups. It is a colorless, volatile, and explosive liquid at room temperature and pressure. It is often used as a precursor in the production of azides, which are widely used in applications like airbags in vehicles.
It is a compound of nitrogen and hydrogen, and is therefore a pnictogen hydride. It was first isolated in 1890 by Theodor Curtius. The acid has few applications, but its conjugate base, the azide ion, is useful in specialized processes.
Properties
- Chemical formula: HN3
- Molar mass: 43.029 g·mol−1
- Appearance: colorless, highly volatile liquid
- Density: 1.09 g/cm3
- Melting point: −80 °C (−112 °F; 193 K)
- Boiling point: 37 °C (99 °F; 310 K)
- Solubility in water: highly soluble
- Solubility: soluble in alkali, alcohol, ether
Preparation
Hydrazoic acid, like its fellow mineral acids, is soluble in water. Undiluted hydrazoic acid is dangerously explosive with a standard enthalpy of formation ΔfHo (l, 298K) = +264 kJ/mol. When dilute, the gas and aqueous solutions (<10%) can be safely prepared but should be used immediately; because of its low boiling point, hydrazoic acid is enriched upon evaporation and condensation such that dilute solutions incapable of explosion can form droplets in the headspace of the container or reactor that are capable of explosion.
Production
The acid is usually formed by acidification of an azide salt like sodium azide. Normally solutions of sodium azide in water contain trace quantities of hydrazoic acid in equilibrium with the azide salt, but introduction of a stronger acid can convert the primary species in solution to hydrazoic acid. The pure acid may be subsequently obtained by fractional distillation as an extremely explosive colorless liquid with an unpleasant smell.
NaN3 + HCl → HN3 + NaCl
Its aqueous solution can also be prepared by treatment of barium azide solution with dilute sulfuric acid, filtering the insoluble barium sulfate.
It was originally prepared by the reaction of aqueous hydrazine with nitrous acid:
N2H4 + HNO2 → HN3 + 2 H2O
With the hydrazinium cation [N2H5]+ this reaction is written as:
[N2H5]+ + HNO2 → HN3 + H2O + [H3O]+
Other oxidizing agents, such as hydrogen peroxide, nitrosyl chloride, trichloramine or nitric acid, can also be used to produce hydrazoic acid from hydrazine.
Occurrences:
Laboratory Preparation: Hydrazoic acid is usually synthesized in the laboratory by reacting sodium azide (NaN₃) with a strong acid (like hydrochloric acid). The reaction produces HN₃ and sodium chloride (NaCl) as a byproduct: NaN3+HCl→HN3+NaCl
In Nature: It is not commonly found in nature, but certain microorganisms and bacteria are known to produce azides as part of their metabolic processes. However, the occurrence of pure hydrazoic acid in natural settings is rare.
In Industry: It is used in the production of azide compounds, which are important in the manufacture of airbags (for automotive safety systems), as well as in the synthesis of other chemicals and pharmaceuticals. Azides, especially sodium azide, are also used in the preparation of explosives and propellants.
Uses
- Precursor for Azides: It’s primarily used in the synthesis of sodium azide and other azide compounds.
- Chemical Reactions: In organic chemistry, hydrazoic acid can be used in various reactions, including the formation of nitrated compounds.
- Airbag Industry: Sodium azide, a compound derived from hydrazoic acid, is used in airbags for vehicles due to its ability to rapidly produce nitrogen gas upon decomposition.
Safety Considerations
Hydrazoic acid is highly toxic and unstable, particularly when it comes in contact with metals like copper, which can cause it to decompose explosively. It can also be harmful when inhaled or ingested, so it must be handled with extreme caution in a controlled environment.