The genetic material of all eukaryotic species is stored in the cell nucleus in the form of DNA. To be utilized, this DNA must first be transcribed into messenger RNA in the cell cytoplasm, then translated into protein by ribosomes, which are little machinery capable of decoding messenger RNA and synthesizing the relevant proteins. However, the rate at which this mechanism occurs is not uniform: it must adapt in order for the protein to adopt the correct structure. Deregulation of the production rate, in fact, causes structural flaws. Proteins that are not appropriately folded clump, become useless, and are frequently harmful to the cell.
A team from the University of Geneva (UNIGE), Switzerland, in collaboration with the University of Hamburg, has demonstrated that the rate of protein synthesis is modulated by regulatory factors that can change the rate of translation of messenger RNA into proteins at will by analyzing the rate of ribosome movement in yeast cells. These findings can be found in the journal Cell Reports.
Once an mRNA has been created, the information contained in its nucleotide sequence is utilized to synthesize a protein via transcription and processing. Because DNA and RNA are chemically and physically similar, DNA can act as a direct template for the production of RNA via complementary base-pairing.
This technology allows us to detect the position of ribosomes at any given time in the cell. It entails destroying any RNA that is not covered by the ribosome at a precise point in order to maintain just the ribosome-protected bits (RPFs). We next sequence these RPFs to determine how many ribosomes were on the mRNA and at what locations at the time.
Olesya Panasenko
Proteins are three-dimensional structures that must interlock or interact with one another in order to function. When there is a structural flaw in a protein, it clumps together, becoming toxic and potentially pathogenic. This effect has been reported in a variety of neurodegenerative disorders, including Alzheimer’s disease and amyotrophic lateral sclerosis. “We already knew that the rate at which proteins are created changes depending on need: sometimes quick, sometimes very slow,” explains Martine Collart, the study’s lead author and professor in the Department of Microbiology and Molecular Medicine at the UNIGE Faculty of Medicine. “However, we didn’t know how this mechanism was controlled at the time.”
Ribosome profiling
To further understand this mechanism, the researchers used a novel and little-known approach called ribosome profiling. “This technology allows us to detect the position of ribosomes at any given time in the cell,” explains Olesya Panasenko, a researcher in Martine Collart’s laboratory and the leader of the ‘BioCode: RNA to Proteins’ Core Facility at the Faculty of Medicine who specialized in this technique. “It entails destroying any RNA that is not covered by the ribosome at a precise point in order to maintain just the ribosome-protected bits (RPFs). Next, we sequence these RPFs to determine how many ribosomes were on the mRNA and at what locations at the time. This shows the translation’s speed and efficiency.”
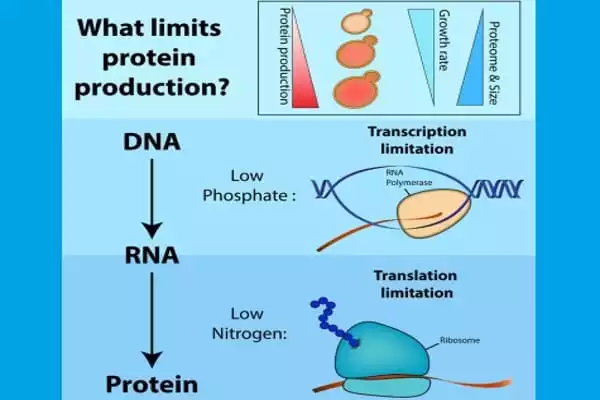
The researchers looked at the pace and dynamics of protein creation in both native yeast cells and genetically engineered yeast cells to see if there were any differences based on the genetic code. Small condensates of RNA and proteins form in the cell during synthesis, limiting the rate of ribosome production. “The creation of these condensates is dependent on the presence or absence of regulatory factors known as Not, which function as decelerators,” Martine Collart adds. In the absence of these factors, the mechanism accelerates in the wrong regions, resulting in aggregated proteins.
A speed regulated by the genetic code
Thus, Not factors bind to the ribosome at specific points during protein synthesis in order to slow down the ribosome during translation by condensing the RNA and the nascent protein. “One can wonder whether this regulating system is influenced by neurodegenerative illnesses or by aging,” the authors speculate. As a result, it is possible that minor disturbances, when added together, will have a major cumulative effect over time.
Depending on where the decoding process begins, an RNA sequence can be translated into one of three possible reading frames. However, only one of an mRNA’s three potential reading frames encodes the essential protein. We’ll see later how a special punctuation signal at the start of each RNA message establishes the correct reading frame at the start of protein synthesis.