Green Rust
Definition
Green rust is an unstable corrosion product typically produced in a low-oxygen environment, such as on rebar in the chlorine-rich environment of seawater. It is a generic name for various green crystalline chemical compounds containing iron(II) and iron(III) cations, the hydroxide (HO−) anion, and another anion such as carbonate (CO2−3), chloride (Cl−), or sulfate (SO2−4), in a layered double hydroxide structure. Green rust is also known as fougerite.

“Fougerite” is a natural rock, whereas the “green rust” is a product formed by a metal corrosion. But in fact it deals with the same compounds. Its formation also corresponds to the depassivation of steel and usually takes place when the chloride concentration or ratio to ions of hydrogen is greater than 1.
Rust is the term given to a large group of iron oxides. Rust can be found when there is unprotected steel or iron that is exposed to the elements. It can form in various colors like yellow, brown, orange and green, which is known as the green rust.
Structure, Production and Properties of Green Rust
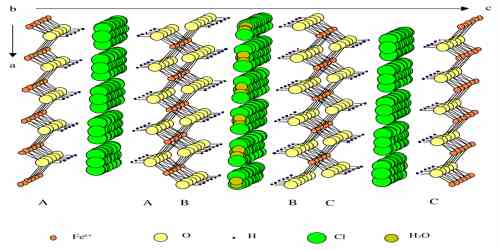
The crystal structure of the Fe(II)-Fe(III) hydroxychloride known as Green Rust one (GR) was investigated by X-ray diffraction and confirmed to be analogous with that of iowaite. It is a rhombohedral crystal [R3m, a=0.3190(1)nm, c=2.385(6)nm] consisting of Fe(OH)2 like-hydroxide sheets which alternate regularly with interlayers composed of Cl ions and H2O molecules and follow the stacking sequence AcBiBaCjCbAk…, where A, B, C are OH layers, a, b, c Fe layers and i, j, k interlayers. By means of transmission Mossbauer spectroscopy analyses at 20K of the solid phases formed during the oxidation of Fe(OH)2 into GR(Cl), it was demonstrated that the composition of the GR compound varied continuously from FeII3FeIII(OH)8Cl\nH2O, with n probably equal to 2, to approximately FeII2.2FeIII(OH)6.4Cl\nH2O.

Several chemical processes produce green rust:
- Electrochemically oxidizing iron plates can form green carbonate rust.
- Green rust may be prepared by bubbling carbon dioxide through a suspension of iron(III) hydroxide Fe(OH)3 in iron(II) chloride FeCl2.
- Green sulfate rust may result from mixing FeCl24H2O and NaOH solution to precipitate Fe(OH)2. Sodium sulfate Na2SO4 is added and the mixture is oxidized in air.
In oxidizing environment, green rust generally turns into Fe3+ oxyhydroxides, namely α-FeOOH (goethite) and γ-FeOOH (lepidocrocite).
Oxidation of the carbonate variety can be retarded by wetting the material with hydroxyl-containing compounds such as glicerol or glucose, even though they do not penetrate the structure. Some variety of green rust is stabilized also by an atmosphere with high CO2 partial pressure.
Green rust compounds can be synthesized at ordinary ambient temperature and pressure, from solutions containing iron(II) cations, hydroxide anions, and the appropriate intercalatory anions, such as chloride, sulfate, or carbonate.
Generally, by oxidation, the ” green rust ” is transformed into made up more stable compound, in which iron is trivalent, lepidocrocite (γ – FeOOH), goethite (α – FeOOH), akaganeite (β – FeOOH) or the magnetite (Fe3 O4) The nature of these products depends on chlorine content and on other factors such as temperature.
Reference: