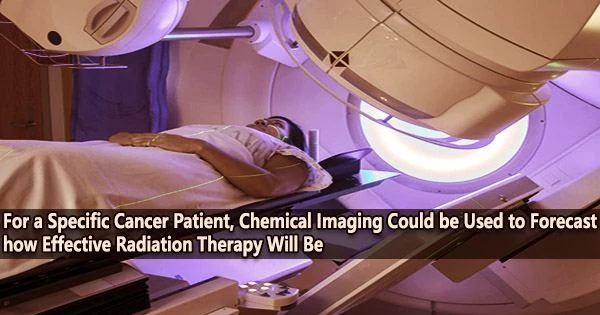
With the use of a novel imaging technique being developed by researchers at the University of Michigan that maps the chemical composition of a patient’s tumor, decisions on cancer treatment may become more specifically tailored to each particular patient.
Currently, the location, size, and aggressiveness of the tumor are the primary factors used to recommend the best cancer treatment options, such as surgery, radiation therapy, or immunotherapy. Biological assays carried out on tissues collected by tumor biopsies and anatomical imaging techniques like MRI, CT, and ultrasound are typically used to obtain this information.
Nonetheless, a treatment’s potential effectiveness is significantly influenced by the chemical environment of a tumor. For instance, radiation therapy is less effective when the tumor tissue has low oxygen levels.
Now, a group of researchers from the University of Michigan and two Italian universities have shown that an imaging system that makes use of unique nanoparticles can produce a high-resolution, real-time chemical map that illustrates the distribution of important compounds in a tumor.
It might result in a strategy to assist medical professionals in providing better cancer therapy suggestions that are personalized for a specific patient.
Their research, published in ACS Nano, reports on the first demonstration of an in vivo chemical imaging method generalizable to any chemical of interest, according to U-M chemistry professor Raoul Kopelman, one of the senior authors on the paper.
The researchers used a method for “chemical imaging” of tissues called photo-acoustic chemical imaging, or PACI.
We thus provide a simple, noninvasive, and inexpensive method to both predict the efficacy of radiation therapy for a given tumor and identify treatment-resistant regions within the tumor’s microenvironment.
Professor Raoul Kopelman
“The novelty of this method is that it is performed in vivo, directly inside the body,” Kopelman said.
The group’s approach was tested in mice that had xenografts tissue taken from a patient’s tumor biopsy implanted. Xenografts made from patients replicate the genetic and biological traits of the patient’s tumor.
In order to target the tumor and detect a specific chemical of biomedical interest, such as oxygen, sodium, or potassium, PACI uses nanoparticles that have been produced in recent years by Kopelman and others.
When infrared laser light that is able to penetrate into the tumor tissues, an ultrasound signal is generated that can be used to map the concentration and distribution of that particular chemical activates this nanosensor.
In a mouse xenograft, the PACI approach could be used to repeatedly monitor the tumor features of a specific patient’s tumor in order to assess the tumor’s chemical environment over time.
“This would allow for optimization of treatment methods for a particular patient precision medicine,” Kopelman said.
Kopelman and colleagues employed the PACI with a nanoparticle targeted to sense oxygen. Following radiation therapy for the mouse tumor, the researchers discovered a significant relationship between oxygen levels in each area of the tumor and the efficiency with which the tumor tissue was destroyed. The lower the local oxygen in the tissue, the lower the local radiation therapy efficacy.
“We thus provide a simple, noninvasive, and inexpensive method to both predict the efficacy of radiation therapy for a given tumor and identify treatment-resistant regions within the tumor’s microenvironment,” Kopelman said.
“Such chemical mapping would help the clinical team prescribe a personalized, optimal treatment for a given patient’s tumor, based on the new diagnostics from the tumor xenograft’s chemical mapping.”
In this research, PACI has been employed in patient-derived xenografts. The ultimate goal would be the ability to make the chemical maps in patients directly.
According to Kopelman, it would be possible to get close to the tumor using fiber optics that could be threaded via the patient’s venous system, as is done during cardiac surgeries. The laser could then activate the nanosensor, but this requires that nanosensors be built for each chemical of interest and approved by the Food and Drug Administration for each one.