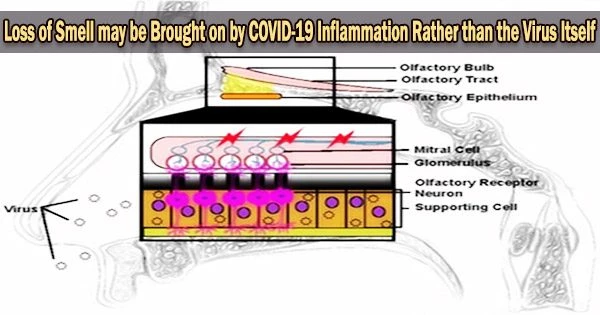
It is thought that inflammation caused by the body’s immune response to the virus may play a role in the loss of smell and taste that some people experience with COVID-19. The inflammation may affect the cells in the nose and throat responsible for detecting smells and tastes.
Loss of smell, or anosmia, is a prevalent and frequently chronic symptom of COVID-19 that can significantly impair a person’s quality of life by making it extremely challenging to taste food, identify airborne risks in the environment, and do other tasks reliant on the sense of smell.
The molecular mechanisms underpinning COVID-mediated anosmia are still largely unknown, despite the condition’s catastrophic effects being well established. Loss of smell and taste can be a symptom of COVID-19, but it can also be caused by other factors such as allergies, sinusitis, and certain medications.
In a study published today in JAMA Neurology, a Johns Hopkins Medicine-led team shows that loss of smell is most likely a secondary consequence of inflammation occurring when the body’s immune system responds to SARS-CoV-2 infection rather than a direct action of the virus.
“As a neuropathologist, I wondered why smell loss is a very common symptom with COVID-19 but not with other respiratory diseases,” says study lead author Cheng-Ying Ho, M.D., Ph.D., associate professor of pathology at the Johns Hopkins University School of Medicine. “So, we decided to dig deeply into the mechanics of smell to see what actually occurs at the cellular level when SARS-CoV-2 invades the body.”
Ho and her team used a control group of 14 people who died from unrelated causes and had no detectable SARS-CoV-2 at the time of their deaths and 23 people who died from COVID-19 to collect tissues from the olfactory bulb at the base of the brain, a region that transmits nerve impulses carrying information about odors.
When we compared the tissues from patients without COVID-19 with those from persons who had been infected with SARS-CoV-2 especially the ones with diminished or complete loss of smell we found that the group with COVID showed more severe vascular injury and far fewer axons in the olfactory bulb.
Cheng-Ying Ho
The structure and properties of the cells, blood vessels, and neurons (nerve cells) within each tissue were examined using both optical and electron microscopy, as well as the quantity of axons (the parts of neurons that send electrical impulses) that were present. Information about sense of smell and taste was obtained from clinical records for three patients and from family interviews for the rest.
Of the 23 COVID-19 patients, three were found to have lost their sense of smell, four to have a decreased sense of smell, and two to have lost both taste and smell. The 14 patients in the control group were not found to have any loss of taste or smell.
Three items were examined in the study of the two groups: the degree of olfactory system neuron degeneration (damage), the loss of olfactory axons, and the severity of microvasculopathy (disease of the small blood vessels). What they found, Ho says, was not unexpected.
“When we compared the tissues from patients without COVID-19 with those from persons who had been infected with SARS-CoV-2 especially the ones with diminished or complete loss of smell we found that the group with COVID showed more severe vascular injury and far fewer axons in the olfactory bulb,” says Ho. “And that didn’t change when we statistically controlled for the impact of age, strongly suggesting that these effects aren’t age-related and therefore, are linked to SARS-CoV-2 infection.”
Ho claims that despite nerve and vascular damage, SARS-CoV-2 particles were not found in the olfactory bulb in the majority of COVID-19 patients, which caught the researchers off guard.
“Previous investigations that only relied on routine pathological examinations of tissue and not the in-depth and ultrafine analyses we conducted surmised that viral infection of the olfactory neurons and olfactory bulb might play a role in loss of smell associated with COVID-19,” she explains.
“But our findings suggest that SARS-CoV-2 infection of the olfactory epithelium leads to inflammation, which in turn, damages the neurons, reduces the numbers of axons available to send signals to the brain and results in the olfactory bulb becoming dysfunctional.”
The following study will be conducted on tissues obtained from patients who passed away from the SARS-CoV-2 Delta and Omicron strains.
“We want to compare any axon damage and bulb dysfunction found in those tissues with what we observed in patients who had the original virus strain,” says Ho. “That way, we’ll be able to better predict if Delta and Omicron are more or less likely to cause loss of smell.”