Researchers at Cincinnati Children’s’ Brain Tumor Center found an unexpected finding while researching a rare but lethal type of brain tumor that may help treat numerous other cancer types that have a YAP fusion protein as a common influencing element.
The findings, published Feb. 2, 2023, in Nature Cell Biology, come from a team led by co-first authors Xiaohua Hu, Ph.D., and Xiaoping Wu, Ph.D., and corresponding author Qing Richard Lu, Ph.D., scientific director of the Brain Tumor Center. 15 additional co-authors from Warsaw, Poland, Shanghai, China, and Cincinnati, Cleveland, and Seattle have also contributed to the work.
The research team was examining why a fraction of kids with ependymoma (EPN), a rare cancer that develops in the brain and down the spinal column, experience disastrous consequences. About 1,100 people a year in the US are diagnosed with EPN, including about 250 children.
These tumors typically form along the spinal cord in adults, and surgery can frequently be used to remove them. Overall, the five-year survival rate for this type of cancer is nearly 84%, according to the National Cancer Institute and the American Society of Clinical Oncology.
However, children with EPN are more likely to develop tumors in the brain. Even though surgically treatable tumors have great survival rates, roughly one-third of children with EPN still develop relapses in spite of receiving chemotherapy, radiation, and surgery. For these children, relapse is nearly always fatal.
The exact causes of EPN tumors are not fully understood. However, children with inherited genetic conditions including Turcot syndrome and neurofibromatosis type 2 (NF2) have an increased risk of developing this form of cancer.
Our findings suggest that targeting biomolecular condensates may eventually prove beneficial for treating relapsed EPN. But first, more research is needed to build upon these findings.
Qing Richard Lu
New data about YAP fusion proteins opens doors
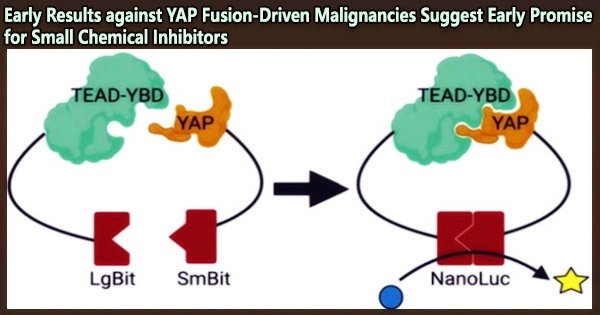
The development of YAP fusion proteins has previously been shown to play a significant role in the growth of a group of EPN cancers. By itself the gene YAP1 produces a protein that blocks the tumor suppressing power of another gene called HIPPO.
But in the last several years, researchers have found a whole family of YAP proteins that develop from YAP1 fusions with other genes in ways that support even more aggressive malignant tumor growth. Subtypes of skin cancer, breast cancer, and various soft-tissue tumors known as sarcomas have YAP fusion proteins found in them.
According to the current study, YAP fusion proteins alter the body’s cancer defenses in a different way than most experts had previously believed. Instead of merely increasing YAP1 activity, these fused YAP proteins cause the nucleus of cells to produce “condensates,” which operate as platforms for brain cell transformation and tumor growth.
By examining the components of these platforms, the scientists discovered that tumor formation can be stopped by inhibiting the activity of BRD4, a gene expression regulator. Known as a transcription factor, this protein sends critical signals to activate other oncogenic genes within nuclear condensates.
The team achieved an early proof-of-concept success when testing their idea in mice that were induced to develop tumors similar to human EPN. The mice treated with two different BRD4 inhibitors survived much longer than mice that did not receive the inhibitors.
What does this mean for people with EPN?
Launching a clinical trial to weigh the advantages and disadvantages of treating persons with EPN with a BRD4 inhibitor will probably require several years of safety testing and related effort. But up until now, a successful surgical procedure was the mainstay of survival for this particular type of cancer. Traditional forms of chemotherapy have shown little to no benefit in relapsed cases.
“Our findings suggest that targeting biomolecular condensates may eventually prove beneficial for treating relapsed EPN,” Lu says. “But first, more research is needed to build upon these findings.”
What does this mean for other types of cancer?
The findings suggests that comparable strategies may be effective in other types of YAP fusion-mediated cancer by demonstrating a successful way for halting the growth of one form of the disease.
“Rather than attempting to prevent the gene fusions from occurring, our study shows that targeting allied molecular processes can reduce the ability of YAP fusion proteins to spur tumor growth,” Lu says.