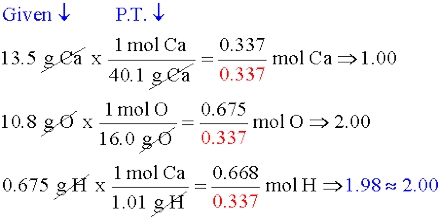
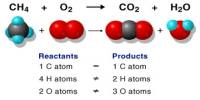
Prime purpose of this lecture is to discuss on Empirical Formula. Empirical Formula is the lowest whole number ratio of elements in a compound. The molecular formula the actual ratio of elements in a compound. For example, if the empirical formula of a compound is C3H8 , its molecular formula may be C3H8 , C6H16 , etc. This lecture also briefly focus on how to Calculate Empirical, Empirical to molecular with examples.
Empirical Formula