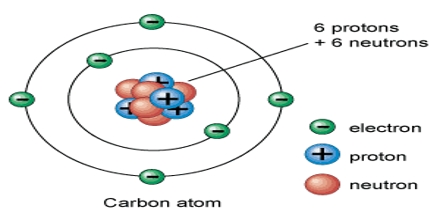
Prime purpose of this lecture is to describe on Electrons in Atoms. Here briefly describe on Rutherford’s model; this model had a problem, it could not explain the chemical properties of atoms. This lecture also describe on Niels Bohr model; he explain: e- are in specific orbits around the nucleus and each orbit contains a fixed amount of energy. Finally describe on Quantum Energy Levels, Periodic Table Arrangement, Atomic Line Spectra and Flame Emission Spectra with examples.
Electrons in Atoms