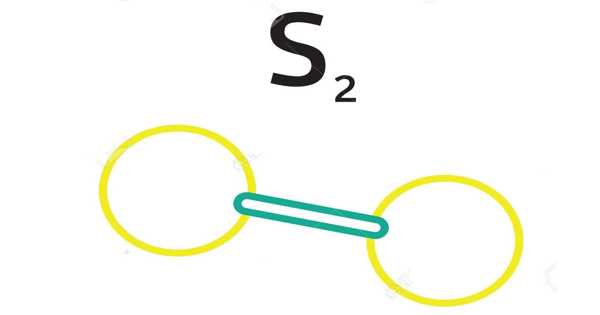
Disulfur is a diatomic molecule with the formula S2. It is a compound composed of two sulfur atoms joined together with an element or radical. Carbon disulfide, CS2, is a sulfide with two sulfur atoms. The generation of excited disulfur molecules by flame chemiluminescence provides an extremely sensitive method for determining sulfur compounds. Disulfur monoxide is unstable in terms of the formation of elemental sulfur and sulfur dioxide; however, it has been generated as a transient species with a sufficient lifetime for complete spectroscopic characterization.
Properties
S2‘s ground state is a triplet: a diradical with two unpaired electrons, similar to O2 and SO. It has an S-S bond length of 189 pm, which is much shorter than S8‘s S-S single bonds, which are 206 pm long. It has a Raman spectrum with a band at 715 cm1. At 1556 cm-1, the corresponding O-O band for O2 can be found. The energy of the S-S bond is 430 kJ/mol, while the energy of the O2 bond is 498 kJ/mol.
Disulfur photodissociates easily, with a mean lifetime of 7.5 minutes in sunlight.

Synthesis
This violet gas is generated by heating sulfur above 720 °C, comprising 99% of the vapor at low pressure (1 mm Hg) at 530 °C.
Disulfur can be produced when an atmosphere of COS is irradiated with UV light using a mercury photosensitizer or when CS2, H2S2, S2Cl2 or C2H4S, PSF3, or COS are irradiated.
Natural occurrence
Gaseous disulfur has been detected emitted from the surface of Jupiter’s moon Io near the Pele volcano.
It is similar to the dioxygen molecule but occurs rarely at room temperature. In hot sulfur vapors, this violet gas is the dominant species. S2 is a minor component of Io’s atmosphere, which is primarily composed of SO2. The double bond rule is commonly used to describe the instability of S2.