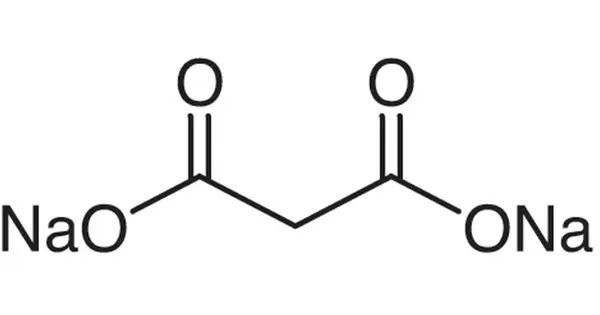
Disodium malonate is a sodium salt of malonic acid with the chemical formula CH2(COONa)2. It is a white crystal soluble in water but not in alcohols, esters or benzene. It appears as a white crystalline powder, soluble in water. It can be prepared from the reaction of sodium hydroxide and malonic acid:
CH2(COOH)2 + 2 NaOH → CH2(COONa)2 + 2 H2O
It acts as a nucleophile in alkylation and condensation reactions due to the active methylene group between the two carbonyls. In biochemistry, malonate is a known competitive inhibitor of succinate dehydrogenase, an enzyme in the citric acid cycle. This inhibition disrupts cellular respiration.
Properties
- Chemical formula: C3H2O4Na2
- Molar mass: 148.025 g/mol
- Boiling point: 659.93K
- Appearance: White crystalline powder
- Solubility in water: High
- Melting point: Decomposes before melting
- pH (aqueous solution): Slightly basic (~8-9)
- Odor: Odorless
- Hygroscopic: Slightly
Preparation
Disodium malonate is typically prepared by neutralizing malonic acid with sodium hydroxide (NaOH):
HOOC-CH2-COOH+2NaOH→NaOOC-CH2-COONa+2H2O
Occurrences
Natural Occurrence:
- Does not occur naturally in significant amounts.
- Malonic acid (its parent acid) is found in small amounts in certain fruits and plants but not commonly in its disodium salt form.
Industrial/Scientific Use:
- Organic synthesis intermediate (used to synthesize barbiturates, vitamins like B1, and other complex molecules).
- Buffer component in biochemical applications.
- Precursor in the malonic ester synthesis.
- Sometimes used in coordination chemistry to create metal-organic frameworks.
Applications
Used primarily in organic synthesis, it serves as a building block in the malonic ester synthesis to produce carboxylic acids. It is also used in research and teaching labs. It is relatively stable under normal conditions but should be handled with care in aqueous or acidic environments.
Safety and Handling
- Hazards: Generally considered low toxicity but can irritate skin and eyes.
- Storage: Store in a cool, dry place; keep container tightly closed.