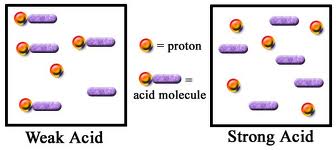
Basic purpose of this article is to Discuss on Strong and Weak Acids. Elements that dissociate completely into ions when put in water are termed as strong electrolytes because the particular high ionic concentration permits an electric current to pass through the solution. Most compounds with ionic bonds behave in this fashion; sodium chloride is an example.
Discuss on Strong and Weak Acids