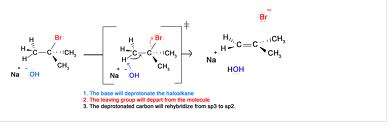
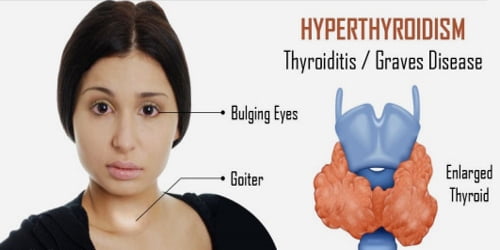
Basic purpose of this article is to Discuss on Mechanism of Elimination Reactions. The halogen‐carbon bond within the alkyl halide is polarized due to the electronegativity difference between the atoms. This polarization can result in the formation of any partial or fully positive charge around the carbon atom. The full or partial positive charge around the carbon atom is delocalized (dispersed) decrease the carbon chain. This particular, in turn, makes the hydrogen atoms that come with these carbons very slightly positive and thus very weakly acidic.
Discuss on Mechanism of Elimination Reactions