Dimethylacetamide (DMAc or DMA) is a strongly polar aprotic solvent with a micro ammonia odor. It is soluble in a wide range of solvents and is miscible with water, aromatics, esters, ketones, alcohols, ether, benzene, chloroform, and other arbitrary solvents. It is the organic compound with the formula CH3C(O)N(CH3)2. It is an acetamide in which the hydrogens bound to the N atom have been substituted by two methyl groups, respectively. In cancer metabolism, a metabolite was discovered. It functions as a metabolite in humans. It is a monocarboxylic acid amide that belongs to the acetamide family. It’s made up of acetamide.
Dimethylacetamide is a solvent and catalyst that is commonly used. Polyacrylonitrile spinning solvent, synthetic resin, and natural resins, formic acid vinyl ester resin, vinyl pyridine copolymer, and aromatic carboxylic acid all use the solvent, which has a high boiling point, flash point, thermal stability, and chemical stability. In organic synthesis, this colorless, water-miscible, high-boiling liquid is widely used as a polar solvent. DMA is miscible with most other solvents, but aliphatic hydrocarbons make it difficult to dissolve.
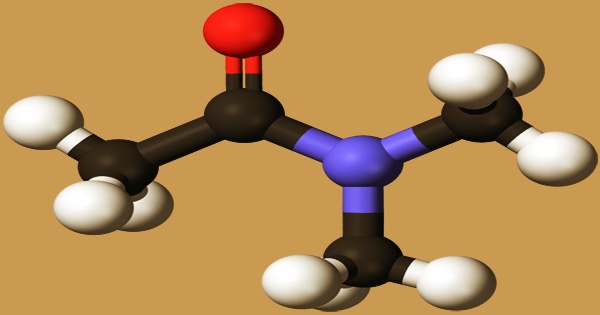
Dimethylacetamide is a colorless, slightly hygroscopic liquid with a faint ammonia odor that is clear, colorless, and slightly hygroscopic. It has a faint ammonia or fishy odor to it. Dimethylamine is commercially generated by reacting it with acetic anhydride or acetic acid. This compound is also obtained by dehydrating the salt of dimethylamine and acetic acid.:
CH3CO2H·HN(CH3)2 → H2O + CH3CON(CH3)2
The catalyst can be used in the urea heating process to manufacture cyanide, sodium halide, and metal cyanide reactions, as well as alkyl acetylene, organic halide, and isocyanate reactions. The reaction of dimethylamine with methyl acetate can also yield dimethylacetamide. Multiple stages of distillation in rectification columns are used to separate and purify the substance. DMA, also known as methyl acetate, is generated with a 99 percent yield.
It has a flash point of 145°F, emits vapors that are heavier than air, and can be harmful if absorbed through the skin. It may irritate the eyes and skin. N,N-dimethylacetamide can also be used as an electrolytic solvent, a coupler solvent in photography, a de paint agent, an organic synthesis raw material, a pesticide, and pharmaceutical raw materials. Styrene separation from the C8 fraction, extraction, distillation, solvent, and so on. Dimethylacetamide’s chemical reactions are similar to those of N,N-disubstituted amides. In the presence of acids, the acyl-N bond is hydrolyzed:
CH3CON(CH3)2 + H2O + HCl → CH3COOH + (CH3)2NH2+Cl−
N,N-dimethylacetamide Department are toxic flammable liquids, and adjustments must be made to ensure that toxic chemicals are stored and transported safely. Dimethylacetamide is widely used in the adhesive industry or as a solvent for fabrics (e.g., polyacrylonitrile, spandex). It’s also used as a reaction medium in the manufacture of pharmaceuticals and plasticizers. Substances that shield the body from the adverse effects of freezing temperatures. It can be used as a solvent for fibers, resins, gums, and electrolytes, as well as a catalyst, paint remover, and a high-purity solvent for crystallization and purification. Dimethylacetamide is also used as an excipient in drugs, e.g., in Vumon (teniposide), Busulfex (busulfan) or Amsidine (amsacrine).
In the pharmaceutical and pesticide industries, it is currently used to synthesize a significant number of antibiotics and pesticides. The catalyst can also be used to make reaction products, electrolytic solvents, paint scavengers, and crystalline solvent adducts and complexes. Like other basic alkyl amides, dimethylacetamide has a low acute toxicity. Hepatotoxicity can result from long-term exposure. It’s also used to dissolve polyacrylonitrile, polyvinyl chloride, polyamides, polyimides, cellulose derivatives, styrenes, and linear polyesters, as well as to make films and fibers.
The use of dimethylacetamide as a vehicle for the parenteral delivery of relatively small peptides has also been studied. Solvents like dimethylacetamide have been shown to affect the size and rate of norfloxacin release from nanoparticles. Because of its reproductive toxicity, dimethylacetamide was designated as a Substance of Very High Concern (SVHC) in the EU in 2011. The European Commission began an investigation in 2014 to limit the use of dimethylacetamide in the EU in accordance with REACH (Registration, Evaluation, Authorization and Restriction of Chemicals).
Dimethylacetamide should be kept in an airtight, light-protected container in a cool, dry place. When stored in sealed containers and under nitrogen, it has a nearly infinite shelf life. Carbon tetrachloride, oxidizing agents, halogenated compounds, and iron are all incompatible with dimethylacetamide. It eats away at plastic and rubber, and it can catch fire if it comes into contact with powerful oxidizers.
Information Sources: