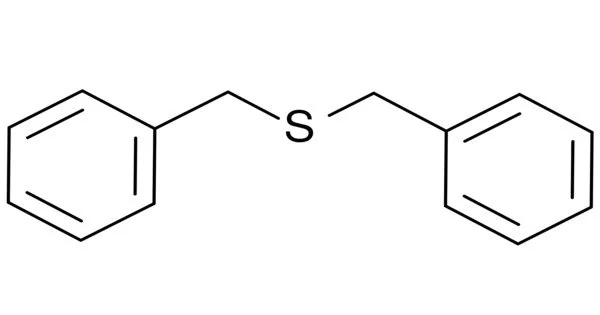
Dibenzyl sulfide is a symmetrical thioether. It has two C6H5CH2- (benzyl) groups connected by a sulfide bridge. It is an organic sulfur compound that belongs to the class of sulfides, which are compounds that include a sulfur atom bound to two organic groups. In the case of dibenzyl sulfide, each sulfur atom is connected to two benzyl groups, which are made up of a benzene ring with a methyl group attached. It is a colorless or white solid that is soluble in nonpolar liquids.
Dibenzyl sulfide has few industrial applications. It could be employed as a chemical intermediary in the synthesis of other organic molecules.
Properties
- Chemical formula: C14H14S
- Appearance: It is a colorless to pale yellow liquid with a characteristic odor.
- Molar mass : 214.33 g·mol−1
- Melting point : 49.5 °C (121.1 °F; 322.6 K)
- Boiling point :322 °C (612 °F; 595 K)
- Density: The density is around 1.06 g/cm³.
- Solubility: It is sparingly soluble in water but soluble in many organic solvents, such as ethanol, diethyl ether, and benzene.
Crystallography
The crystal structure of the solid is of the orthorhombic system with space group Pbcn; number 60. The unit cell dimensions are a=13.991 Å b=11.3985 Å c 7.2081 Å. The molecules in the gas take the same form as in the solid with a C2 symmetry.
Production
Benzyl sulfide is commercially manufactured by treating potassium sulfide with benzyl chloride, followed by distillation of the product. It is also obtainable by desulfurization of dibenzyldisulfide with phosphine reagents.
Application
Dibenzyl sulfide is utilized in a variety of commercial applications, including as a chemical intermediary in the synthesis of other organic compounds and as a food flavoring ingredient. It is also found in some natural sources, such as certain plants and vegetables, and contributes to their scent and flavor.
Hazards
Dibenzyl sulfide, like many organic substances, can be hazardous to one’s health and safety if handled incorrectly. When working with this chemical, it is critical to follow standard safety precautions, such as wearing personal protection equipment and conducting reactions in a well-ventilated location.