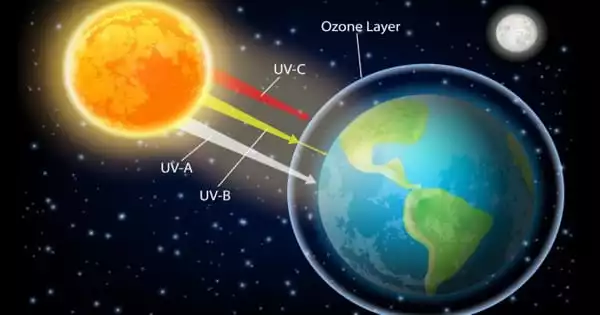
Increased UV radiation levels at the Earth’s surface are caused by ozone layer depletion, which is harmful to human health. Increases in certain types of skin cancer, eye cataracts, and immune deficiency problems are among the negative effects.
When chlorine and bromine atoms come into touch with ozone molecules in the stratosphere, they destroy them. Before it is evacuated from the stratosphere, one chlorine atom can damage over 100,000 ozone molecules. Ozone can be destroyed faster than it can be formed.
The Earth’s atmosphere is divided into strata. The troposphere is the layer closest to the surface, and it stretches from the Earth’s surface up to around 10 kilometers. The stratosphere contains the ozone layer, which is located above the troposphere (10 km to about 50 km high). Stratospheric ozone provides natural protection for all life on Earth by insulating it from damaging ultraviolet-B (UV-B) radiation. UV-B radiation is toxic to humans, animals, and plants. Certain industrial chemicals, such as ozone-depleting refrigerants, halons, and methyl bromide, a lethal insecticide commonly used on crops, are destroying the ozone layer.
When the stratosphere is very cold, the damage from ozone depletion becomes considerably worse. This has been the case for the last two years, resulting in significant ozone depletion. This past winter, ozone depletion in the Northern Hemisphere reached unprecedented levels. Ozone levels in the western United States are likewise declining at a rate of 3-4 percent every decade. Even if all of our efforts to reduce harmful emissions are successful, the ozone layer is not likely to recover until at least 2020.
Ozone depletion occurs in several parts of the Earth’s ozone layer, most notably in the polar regions. NOAA scientists have been to Antarctica to research the ozone depletion that has been occurring there since the late 1970s. Soon after the reported finding of the ozone hole in 1986, Aeronomy Lab (now ESRL) scientist Dr. Susan Solomon led a team of 16 scientists to the conclusion that human-produced trace gases like chlorine and bromine were creating the ozone hole.
This unique data from the South Pole station clearly depicts the annual growth of springtime Antarctic ozone depletion over the last two decades. Images made from data collected by the NASA TOMS satellite and the NOAA SBUV-2 instruments aboard NOAA satellites provide another perspective on ozone depletion at the South Pole. Continued monitoring is required to confirm the projected recovery of the ozone layer.
Arctic Ozone
During the late winter and spring months (January – April), the Arctic ozone layer suffers significant degradation. However, the maximum depletion is often less severe than that observed in the Antarctic, with no significant and recurring ozone hole occurring in certain industrial processes and consumer items that result in the atmospheric production of ozone-depleting chemicals. These gases contain chlorine and bromine atoms, both of which are known to be damaging to the ozone layer. These gases eventually reach the stratosphere, where they are broken apart, releasing ozone-depleting chlorine atoms.
Another key topic of study for NOAA scientists is methyl bromide. It is mostly employed as an agricultural fumigant, but it also contributes significantly to bromine levels in the atmosphere. Although certain ozone-depleting gases are emitted by natural sources, human-caused emissions outnumber those from natural sources. NOAA researchers measure ozone-depleting substances in the lower and upper atmosphere on a regular basis and seek to account for reported variations. Ozone-depleting gases are being replaced in human activities by ‘ozone-friendly’ gases as a result of international laws.
The world’s population has a say in policies that limit ozone-depleting gas emissions. The Montreal Protocol on Substances that Deplete the Ozone Layer was established by the world community in 1987. Since the first treaty’s ratification, ongoing assessments and revisions have been carried out. The Protocol’s effectiveness has been attributed in part to scientific updates on the science and observation of ozone depletion made during the last 15 years. NOAA researchers from various laboratories have been involved in all of these scientific updates, as well as in the preparation of outreach publications to communicate the science of ozone depletion to the general public.