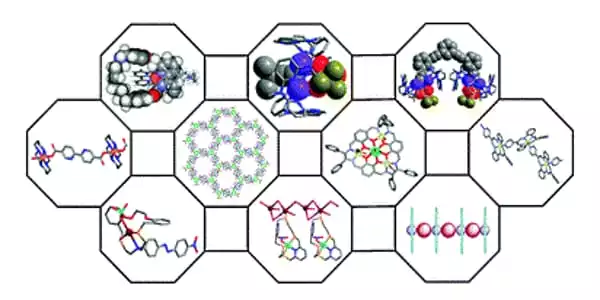
To form highly fluorescent metal chelates, a metal ion is combined with a weakly fluorescent or nonfluorescent compound containing chelate-forming, electron-donating functional groups. Researchers explain how large cyclic molecules can be used to synthesize large metallic complexes containing two or more metal atoms.
Polymetallic complexes are formed when two or more metal atoms combine with organic molecules to form larger, more complex molecular structures. These complexes are used in the development of new catalysts, molecular magnets, and sensors, among other things. Polymetallic complexes were previously synthesized through the trial-and-error method of combining metal ions with organic ligands, resulting in unpredictable compounds.
The modern approach makes use of macrocycles, which are organic molecules with a ring structure. The interior space of macrocyclic molecules can be used to anchor a polymetallic complex during its formation, allowing for the reproducible synthesis of predictable end products.
Researchers describe how large cyclic molecules can be used for the synthesis of big metallic complexes with two or more metal atoms.
Shigehisa Akine of Kanazawa University, Mark MacLachlan of the University of British Columbia (UBC) and NanoLSI (Kanazawa University), and Mohammad Chaudhry, a UBC Ph.D. student, have now published a comprehensive overview of the synthesis of polymetallic complexes via the macrocycle route, which also discusses how certain properties of a complex can be tuned by changing the composition of the macrocycle used.
The scientists begin by discussing the field’s origins. It was demonstrated in the 1970s that [2+2] macrocycle dinuclear complexes could be formed using a relatively simple organic compound with the molecular formula C9H8O3 as the building block. These dinuclear complexes are made up of two metal atoms that are arranged in an organic ‘web’ with two-fold symmetry. Similar Robson macrocycles, as they are known, can be created using six metals, with the overall [3+3] structure having three-fold (triangular) symmetry. Robson macrocycles are still being studied today, but the method is somewhat unpredictable.
Cation separation is aided by complexation with auxiliary ligands. Migration times vary according to the degree of complexation. Thus, the type and concentration of the complexing agent, pH, ionic strength, viscosity, and so on can all influence migration.
The researchers go on to explain how [2+2] (two-fold symmetry) and [3+3] (triangular symmetry) macrocycles are also used in modern designs. Because of their potential as single-molecule magnets – molecules that exhibit (para)magnetism – the [3+3] compounds are currently being actively researched. The magnetic properties of the resulting molecules can be tuned using macrocycles by varying cluster sizes and compositions. The [2+2] complexes are known to have cavities that can be exploited to form unique clusters.
The ‘Pacman macrocycles,’ which are made up of ligands with a cleft, are another intriguing class of multimetallic structures. Small metal-ligand-metal molecules can be captured and activated using this geometry. Pacman macrocycles with two uranium atoms have been extensively studied in the context of nuclear waste processing in this context. Akine, MacLachlan, and Chaudhry also demonstrate that, more broadly, chemists have succeeded in creating several structurally and chemically distinct polymetallic complexes by using Pacman ligands.
The researchers’ final type of macrocycle is one with pyridine rings (pyridine is similar to benzene, with one C-H unit replaced by nitrogen). Pyridine-ring macrocycles have a high degree of flexibility and can be used to synthesize a wide range of complex multimetallic structures; the authors provide numerous examples of silver-containing complexes.
The scientists conclude their review with an outlook on this fascinating research area. They specifically mention that mimicking the activity of naturally occurring clusters in living systems is likely to be a future trend. Indeed, polymetallic complexes play critical roles in important reactions such as the reduction of nitrogen to ammonia and the oxidation of carbon monoxide to carbon dioxide. According to Chaudhry et al., “the potential selectivity and control rendered by the macrocycle-templating method is especially appealing for consistent, reproducible production of functional multimetallic clusters.”
Complexation is a critical process that determines whether mineral solubility limits are reached, the amount of adsorption that occurs, and the redox state of the water. Chemicals and ligands can also form stable, soluble complexes. Synthetic chelating agents (such as ethylenediamine), partially oxidized biodegradation products (such as acids), and natural humic materials could all be ligands in leachates.