Since lithium-ion batteries (LIBs) were commercially commercialized, human lives have undergone a revolution. However, the rising energy requirements of contemporary portable electronics necessitate the use of batteries with high energy densities.
Directly using Li metal as an anode is considered a promising strategy due to its ultra-high capacity (3860 mA h g-1) and low negative electrochemical potential (- 3.04 V versus the standard hydrogen electrode).
However, due to low cyclability, significant safety concerns, and limited rate capability, utilizing Li metal is currently not an option. The development of a thin solid-electrolyte interphase (SEI) layer is a significant problem.
The SEI layer is formed when Li metal reacts with the electrolyte. Because of its instability and the significant volume change of lithium metal, the SEI layer frequently fails.
When SEI breaks down, unreacted Li is exposed. Unreacted Li then combines with liquid electrolytes to create more SEI, which leads to cell failure during the cycling process.
Other major challenges preventing the use of Li metal as anodes include uneven nucleation and the development of needle-like Li dendrites. These arise from a localized increase in current density, and when they eventually pierce the separator, they pose a safety risk. To prevent the creation of lithium dendrites, an efficient technique must be developed.
Recently, Prof. Zhifeng Zheng’s team from Xiamen University published a manuscript entitled “Homogenous metallic deposition regulated by abundant lithiophilic sites in nickel/cobalt oxides nanoneedle arrays for lithium metal batteries” in Journal of Energy Chemistry.
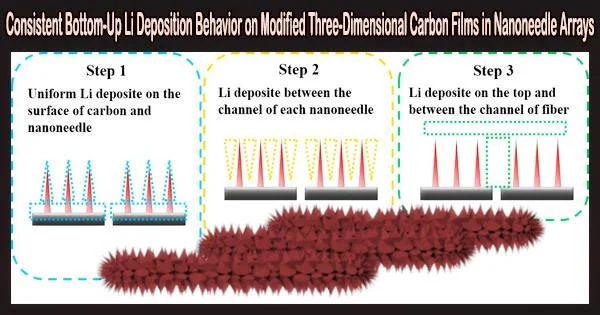
To achieve reversible lithium stripping/plating cycling at high current density, lignin-derived carbon film was produced with rock salt type NiO and CoO nanoneedle arrays. The 3D networks’ logical design contributes significantly to the homogenous Li-ion flux and reduction of local current density.
The Li dendritic development and “dead” Li creation are inhibited by the numerous lithiophilic spots in the film. The novel three-dimensional nanoneedle arrays can permit uniform bottom-up Li deposition. Because of its unique structure, the NCO-CNF electrode displayed stable lithium stripping/plating cycling up to 4000 h.
In addition, the surface of the nanoneedles and the surrounding channel had the strongest lithiophilic qualities, followed by the bottom of the special structure. A NCO-CNF/Li|LFP full cell with an N/P ratio of three exhibits impressive reversibility at 0.5 C up to 80 cycles.
In conclusion, the work offers a logical plan for creating lithiophilic substrates that will encourage uniform metallic deposition, which may be a practical way to address dendrite problems in lithium metal batteries of the future.