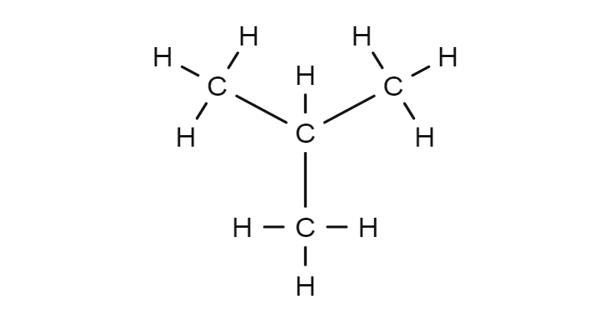
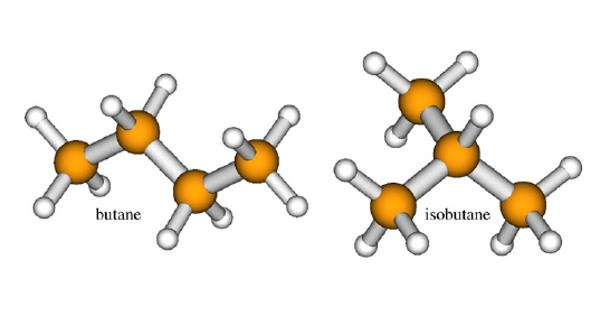
Isobutane, also known as i-butane, 2-methylpropane or methylpropane, is a chemical compound with molecular formula HC(CH3)3. It is a colorless gas with a faint petroleum-like odor. It is an isomer of butane. Isobutane is a colourless, odourless gas. It is shipped as a liquefied gas under its vapor pressure. It is the simplest alkane with a tertiary carbon. It is an alkane that is propane substituted by a methyl group at position 2.
Isobutane is used as a precursor molecule in the petrochemical industry, for example in the synthesis of isooctane. It has a role as a food propellant and a refrigerant. It is a highly demanded chemical in the refinery industry for the production of alkylates, and methyl tert-butyl ether (MTBE), both important additives for reformulated gasolines.
Production Process
Isobutane production is converted from butane (n-butane) in a process called isomerization. The isobutane production process rearranges the atoms into a different molecular configuration.
The component atoms are the same but are arranged in a different geometric structure. This isomerization happens in something called a butamer unit and includes the use of platinum or another metal catalyst.
In this isobutane production process, only some of the butane is actually converted to isobutane. After the butamer process, the output mixture goes through a fractionator or deisobutanizer tower that separates the unconverted butane from the isobutane production.
Uses
Isobutane is the principal feedstock in alkylation units of refineries. It is indeed a raw material for the production of MTBE, synthetic rubber, and many other organic products. Using isobutane, gasoline-grade “blendstocks” are generated with high branching for good combustion characteristics. Typical products from isobutane are 2,4-dimethylpentane and especially 2,2,4-trimethylpentane. It is used to make isooctane, a high octane gasoline component, which increases the octane rating and anti-knock properties of gasoline.
- Solvent: In the Chevron Phillips slurry process for making high-density polyethylene, isobutane is used as a diluent.
- Precursor to tert-Butyl hydroperoxide: Isobutane is oxidized to tert-Butyl hydroperoxide, which is subsequently reacted with propylene to yield propylene oxide.
- Miscellaneous uses: Isobutane is also used as a propellant for aerosol cans. It is used as part of blended fuels, especially common in fuel canisters used for camping.
Toxicity
Contact with the liquid can cause frostbite. It is easily ignited. It is very flammable and gas/air mixtures can be explosive. The vapors are heavier than air.
Information Source: