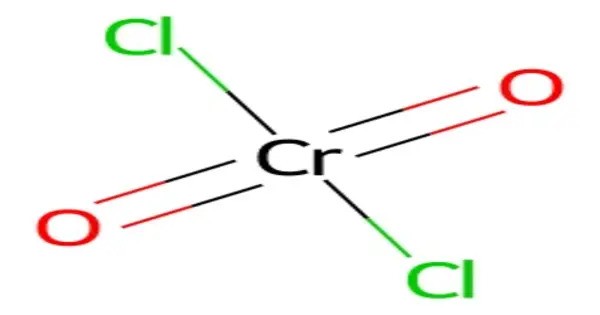
Chromyl chloride is an inorganic compound with the formula CrO2Cl2. It is a strong oxidizer, used in the Etard reaction to oxidize toluene derivatives to aldehydes. It is a reddish brown compound that is a volatile liquid at room temperature, which is unusual for transition metal compounds. It is corrosive, attacking organic materials and metals, and must be handled under dry conditions due to its moisture sensitivity. It is the dichloride of chromic acid.
Its structure features a tetrahedral chromium center with two oxo and two chloro ligands, contributing to its high reactivity. Safety concerns include its toxicity, carcinogenicity, and environmental hazards due to chromium(VI). Proper storage in sealed, dry containers and handling in fume hoods are essential. Its use is regulated due to health risks.
Properties
Chromyl chloride is a dark red, volatile liquid with a pungent odor, used primarily as an oxidizing agent in organic chemistry. It has a molecular weight of 154.9 g/mol, a boiling point of 117°C, and a melting point of -96.5°C. Highly reactive, it fumes in air, releasing toxic chromium(VI) and hydrochloric acid vapors upon hydrolysis.
- Chemical formula: CrO2Cl2
- Molar mass: 154.9008 g/mol
- Appearance: Blood-red fuming liquid, similar to bromine
- Odor: Musty, burning, acrid
- Density: 1.911 g/mL, liquid
- Melting point: −96.5 °C (−141.7 °F; 176.7 K)
- Boiling point: 118.5 °C (245.3 °F; 391.6 K)
- Solubility in water: Reacts with water
- Vapor pressure: 20 mmHg (20 °C)
Synthesize
It is synthesized by reacting chromium(VI) oxide (CrO₃) with chloride salts (e.g., NaCl) in the presence of sulfuric acid, often via the chromyl chloride test to detect chloride ions in salts, forming the characteristic red vapor.
Preparation
Chromyl chloride can be prepared by the reaction of potassium chromate or potassium dichromate with hydrogen chloride in the presence of concentrated sulfuric acid, followed by distillation.
K2Cr2O7 + 6 HCl → 2 CrO2Cl2 + 2 KCl + 3 H2O
The sulfuric acid serves as a dehydration agent.
It can also be prepared directly by exposing chromium trioxide to anhydrous hydrogen chloride gas.
CrO3 + 2 HCl ⇌ CrO2Cl2 + H2O
Occurrences
Natural Occurrence: Chromyl chloride does not occur naturally in significant quantities due to its high reactivity and instability in the environment. It is primarily a synthetic compound.
Used in the chromyl chloride test to detect chloride ions in inorganic salts, producing red fumes of CrO₂Cl₂ when heated with potassium dichromate and sulfuric acid.
Usage
Industrial/Research Use: Found in controlled laboratory settings or industrial processes involving chromium chemistry, such as catalysis or organic synthesis. Not persistent in nature due to its reactivity.
Test for the presence of chlorides: The chromyl chloride test involves heating a sample suspected to contain chlorides with potassium dichromate and concentrated sulfuric acid. If a chloride is present then chromyl chloride forms which is indicated by the evolution of red smoke. No similar compound is formed in the presence of fluoride, bromide, iodide, or cyanide, making this test specific to chlorides.
Reagent for oxidation of alkenes: Chromyl chloride oxidizes internal alkenes to alpha-chloroketones or related derivatives. It will also attack benzylic methyl groups to give aldehydes via the Étard reaction. Dichloromethane is a suitable solvent for these reactions.
Safety Note
Chromyl chloride is toxic, carcinogenic, and a strong irritant. It should be handled in a fume hood with proper protective equipment. Its fumes can cause severe respiratory and skin irritation.